Page 235 - Thermodynamics of Biochemical Reactions
P. 235
Apparent Equilibrium Constants 235
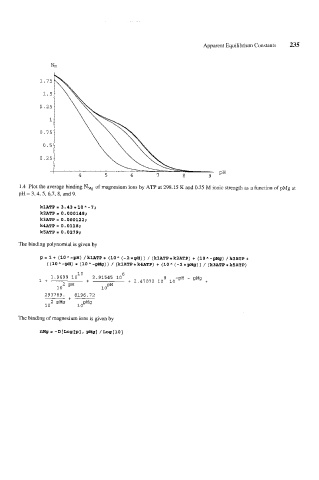
1.4 Plot the average binding m~~ of magnesium ions by ATP at 298.15 K and 0.25 M ionic strength as a function of pMg at
pH = 3,4,5, 6,7, 8, and 9.
kl ATP = 3.43 * 10 " - 7 i
kZATP= O.OOOl48;
k3ATP=0.000122;
k4ATP= 0.0118;
kSATP= 0.0279;
The binding polynomial is given by
B= 1+ (10*-pH) /klATP+ (10" (-2*pH)) / (klATP*k2ATP) + (lOA-pMg) /k3ATP+
((lO"-pH) * (lO"-Mg)) / (klATP*k4ATP) + (10" (-2+pMg)) / (k3ATPekSATP)
293789. 8196.72
+-
102 PMg
1 OPMg
The binding of magnesium ions is given by
nMg = -D[Log[p] r PMg] /LOg[lO]