Page 236 - Thermodynamics of Biochemical Reactions
P. 236
236 Mathematica Solutions to Problems
18873.6
) /
1 OPM9
10 6
1.9699 10 2.91545 10
((1 + t f
lo2 pH 1 OPH
8 -pH - pMg 293789. 8196.72
2.47072 10 10 +-t- ) Log[lOI
102 PMg 10PMg
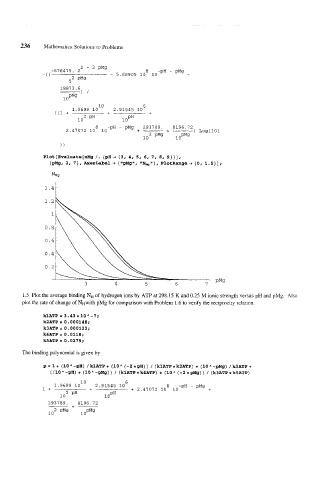
PlOt[Evaluate[nMg /. {pH+ {3, 4, 5, 6, 7, 8, 9)}],
bMg, 2 I 7 } , AxesLabel + { "pMg", *INm I' } , PlotRange + { 0, 1.5) ] ;
NMg
1.41
3 4 5 6 7
1.5 Plot the average binding NH of hydrogen ions by ATP at 298.15 K and 0.25 M ionic strength versus pH and pMg. Also
plot the rate of change of WH With pMg for comparison with Problem 1.6 to verify the reciprocity relation.
klATP = 3.43 * 10 -7;
k2ATP=0.000148;
k3ATP=0.000122;
k4ATP=0.0118;
k5ATP= 0.0279;
The binding polynomial is given by
p = 1 + (10 A -pH) / klATP + (10 (-2 *pH) / (klATP*k2ATP) + (10 A -pMg) / k3ATP +
)
((lO"-pH) * (lO"-pMg)) / (klATP*k4ATP) + (10" (-2*pMg)) / (k3ATP*kSATP)
293789. +- 8196.72
2 PMg PMg
10