Page 238 - Thermodynamics of Biochemical Reactions
P. 238
238 Mathematica Solutions to Problems
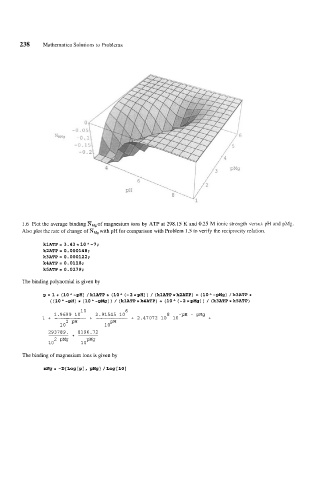
1.6 Plot the average binding mM,of magnesium ions by ATP at 298.15 K and 0.25 M ionic strength versus pH and pMg
Also plot the rate of change of NMg with pH for comparison with Problem 1.5 to verify the reciprocity relation.
klATP = 3.43*10"-7;
k2ATP=0.000148;
k3ATP=0.000122;
k4ATP= 0.0118;
kSATP=0.0219;
The binding polynomial is given by
g=l+ (lOA-gH) /klATP+ (10" (-2*gH)) / (klATP*k2ATP) + (10"-gMg) /k3ATP+
((lOA-gH) * (lOA-pMg)) / (klATP*k4ATP) + (10" (-2*gMg)) / (k3ATP*kSATP)
293789. 8196.72
+-
The binding of magnesium ions is given by