Page 233 - Thermodynamics of Biochemical Reactions
P. 233
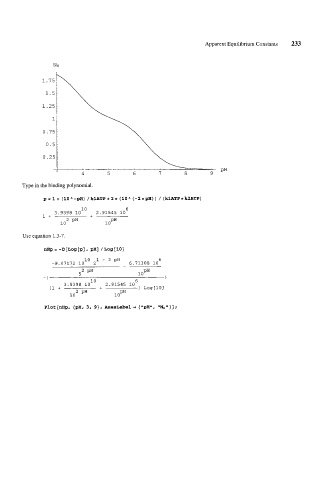
Apparent Equilibrium Constants 233
Type in the binding polynomial.
P=l+ (10”-pH) /klATP+2*(10A(-2*pH)) /(klATP*k2ATP)
10 6
3.9398 10 2.91545 10
1+ +
2 PH
10 loPH
Use equation 1.3-7.
nHP=-D[LOg[P]r PHI /LOg[lOI
10 1 - 2 pH 6
-9.07172 10 2 - 6.71308 10
52 PH PH
10
-I \
\
6
3.9398 10 lo 2.91545 10
(1 + ) Log[lOI
PH
lo2 pH 10