Page 248 - Thermodynamics of Biochemical Reactions
P. 248
248 Matheniatica Solutions to Problems
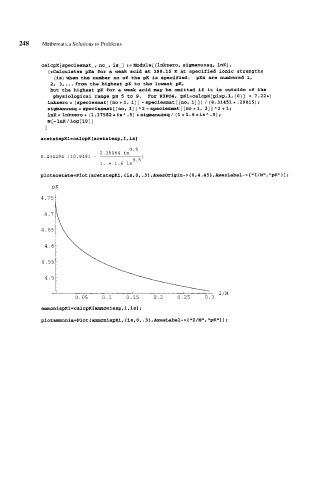
CalCpK[SpeCieSmat-, no-, is-] := Module[{lnkzero, sigmanuzsg, InK),
(*Calculates pKa for a weak acid at 298.15 K at specified ionic strengths
(is)when the number no of the pK is specified. ~Ks are numbered 1,
2, 3,...frcrm the highest pK to the lowest pK,
but the highest pK for a weak acid may be omitted if it is outside of the
physiological range pH 5 to 9. For H3P04, gK1=CalCpK[giSp,l,{O}] = 7.22*)
lnkzero= (speciesmat[ [no+l, 113 -speciesmat[[no, 133) / (8.31451e.29815);
sigrnanuzsq = speciesmat [ [no, 31 ] A 2 - speciesmat [ [no + 1, 31 ] A 2 + 1;
1nK = lnkzero + (1.17582 *is A .5) * sigmanuzsg/ (1 + 1.6 *is A .5) ;
N [ - 1nK / Log [ 101 ]
1
plotacetate-Plot [acetatepKl, Cis, 0, .3 1 ,Axesorigin-> (0,4.45} ,AxesLabel-> { "I/M", "pK"} 1 ;
I /M
0.05 0.1 0.15 0.2 0.25 0.3