Page 251 - Thermodynamics of Biochemical Reactions
P. 251
Apparent Equilibrium Constants 251
F
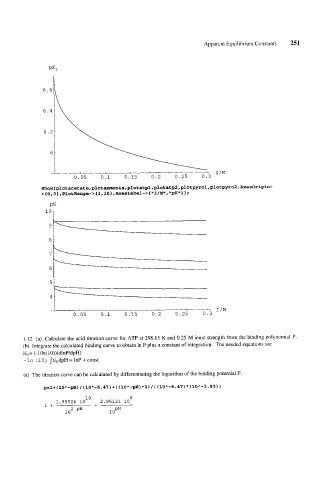
1.12 (a) Calculate the acid titration curve for ATP at 298.15 K and 0.25 M ionic strength from the binding polynomial P.
(b) lntegrate the calculated binding curve to obtain In P plus a constant of integration. The needed equations are
NH= (-l/ln(lO))(dlnP/dpH)
-In (10) INHdpH = InP + const
(a) The titration curve can be calculated by differentiating the logarithm of the binding potential P.
~~1+(10"-~H)/(10*-6.47)+((1OA~~H)"2)/((1OA~6~47)*~~OA~3~83~~
10 6
1.99526 10 + 2.95121 10
1r
I 8
2 PH
10 1 OPH