Page 329 - Thermodynamics of Biochemical Reactions
P. 329
Systems of Biochemical Reactions 329
{-1, 2, -2, -2, 2, -2, 0, 0, 0, 0, 0, 0, 0, 0, 0, 21
mke~[nu2.{1,1,1,1,lr2,2,2,2,2,2},n~es21
2 ADP + Glc + 2 NADox + 2 Pi -> 2 ATP + 2 NADred + 2 Pyr
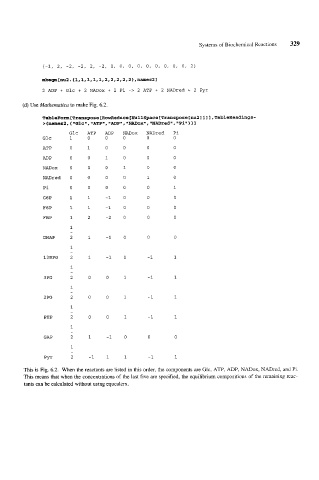
(d) Use Mathematica to make Fig. 6.2.
TableForm[Transpose[RowReduce[~llSpacetTrans~ose[nu2l11l,TableHeadings-
>{names2, {iiGlcn,ilATPii, iiADPii,iiNADoxll,iINADredLII, 11
llPiill
Glc ATP ADP NADox NADred Pi
Glc 1 0 0 0 0 0
ATP 0 1 0 0 0 0
ADP 0 0 1 0 0 0
NADox 0 0 0 1 0 0
NADred 0 0 0 0 1 0
Pi 0 0 0 0 0 1
G6P 1 1 -1 0 0 0
F6P 1 1 -1 0 0 0
FBP 1 2 -2 0 0 0
1
-
DHAP 2 1 -1 0 0 0
1
-
13BPG 2 1 -1 1 -1 1
1
-
3 PG 2 0 0 1 -1 1
-
2 PG 2 0 0 1 -1 1
1
-
PEP 2 0 0 1 -1 1
1
-
GAP 2 1 -1 0 0 0
1
-
PYr 2 -1 1 1 -1 1
This is Fig. 6.2. When the reactants are listed in this order, the components are Glc, ATP, ADP, NADox, NADred, and Pi.
This means that when the concentrations of the last five are specified, the equilibrium compositions of the remaining reac-
tants can be calculated without using equcalcrx.