Page 218 - Uninterruptible Power Supplies
P. 218
Batteries
216 Chapter Seven
Percentage (%)
110
100
90
80
70
Available capacity
60
Charge efficiency
50
40
30
Charge at constant current
20
(current 0.2 C5 A)
10
0
0 20 40 60 80 100 120 140
Charged capacity (% C5 Ah)
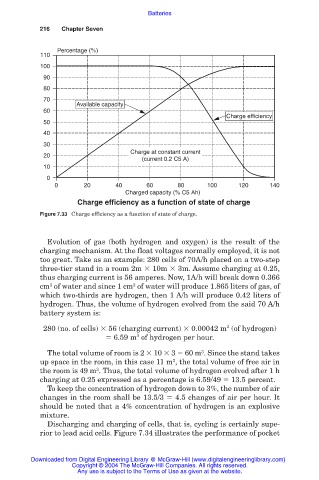
Charge efficiency as a function of state of charge
Figure 7.33 Charge efficiency as a function of state of charge.
Evolution of gas (both hydrogen and oxygen) is the result of the
charging mechanism. At the float voltages normally employed, it is not
too great. Take as an example: 280 cells of 70A/h placed on a two-step
three-tier stand in a room 2m 10m 3m. Assume charging at 0.25,
thus charging current is 56 amperes. Now, 1A/h will break down 0.366
3
3
cm of water and since 1 cm of water will produce 1.865 liters of gas, of
which two-thirds are hydrogen, then 1 A/h will produce 0.42 liters of
hydrogen. Thus, the volume of hydrogen evolved from the said 70 A/h
battery system is:
3
280 (no. of cells) 56 (charging current) 0.00042 m (of hydrogen)
3
6.59 m of hydrogen per hour.
3
The total volume of room is 2 10 3 60 m . Since the stand takes
up space in the room, in this case 11 m , the total volume of free air in
3
3
the room is 49 m . Thus, the total volume of hydrogen evolved after 1 h
charging at 0.25 expressed as a percentage is 6.59/49 13.5 percent.
To keep the concentration of hydrogen down to 3%, the number of air
changes in the room shall be 13.5/3 4.5 changes of air per hour. It
should be noted that a 4% concentration of hydrogen is an explosive
mixture.
Discharging and charging of cells, that is, cycling is certainly supe-
rior to lead acid cells. Figure 7.34 illustrates the performance of pocket
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.