Page 219 - Uninterruptible Power Supplies
P. 219
Batteries
Batteries 217
Cycles
10.000
9.000
8.000 Cycle life versus depth of discharge expressed as
7.000
a percentage of the rated capacity
6.000
5.000
4.000
Temperature +20°C
3.000
2.000
1.000
0
10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
Depth of discharge
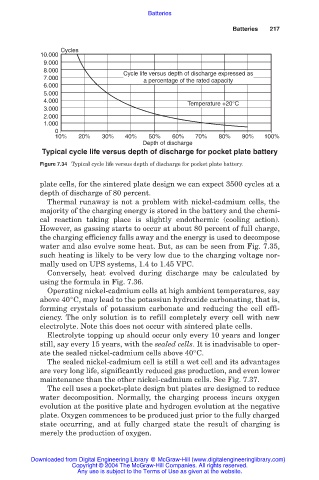
Typical cycle life versus depth of discharge for pocket plate battery
Figure 7.34 Typical cycle life versus depth of discharge for pocket plate battery.
plate cells, for the sintered plate design we can expect 3500 cycles at a
depth of discharge of 80 percent.
Thermal runaway is not a problem with nickel-cadmium cells, the
majority of the charging energy is stored in the battery and the chemi-
cal reaction taking place is slightly endothermic (cooling action).
However, as gassing starts to occur at about 80 percent of full charge,
the charging efficiency falls away and the energy is used to decompose
water and also evolve some heat. But, as can be seen from Fig. 7.35,
such heating is likely to be very low due to the charging voltage nor-
mally used on UPS systems, 1.4 to 1.45 VPC.
Conversely, heat evolved during discharge may be calculated by
using the formula in Fig. 7.36.
Operating nickel-cadmium cells at high ambient temperatures, say
above 40°C, may lead to the potassiun hydroxide carbonating, that is,
forming crystals of potassium carbonate and reducing the cell effi-
ciency. The only solution is to refill completely every cell with new
electrolyte. Note this does not occur with sintered plate cells.
Electrolyte topping up should occur only every 10 years and longer
still, say every 15 years, with the sealed cells. It is inadvisable to oper-
ate the sealed nickel-cadmium cells above 40°C.
The sealed nickel-cadmium cell is still a wet cell and its advantages
are very long life, significantly reduced gas production, and even lower
maintenance than the other nickel-cadmium cells. See Fig. 7.37.
The cell uses a pocket-plate design but plates are designed to reduce
water decomposition. Normally, the charging process incurs oxygen
evolution at the positive plate and hydrogen evolution at the negative
plate. Oxygen commences to be produced just prior to the fully charged
state occurring, and at fully charged state the result of charging is
merely the production of oxygen.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.