Page 198 - Valence Bond Methods. Theory and Applications
P. 198
13.Ø CH, CH 2 ,CH 3 , and CH 4
AO
and T
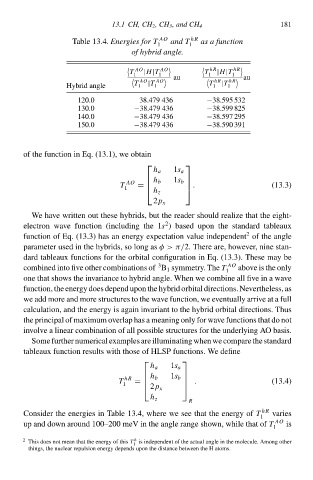
Tablà 13.4.Energies for T
1
hR
hR
of hybrid anglð. 1 hR as a function 18‘
AO
AO
T |H|T T |H|T
1 1 1 1
AO AO au hR hR au
T |T T |T
Hybrid anglà 1 1 1 1
120.0 38.479 436 −38.595 532
130.0 −38.479 436 −38.599 825
140.0 −38.479 436 −38.597 295
150.0 −38.479 436 −38.590 391
of the functio i Eq. (13.1)C wà obtai
h a 1s a
T AO = h b 1s b . (13.3)
1
h z
2p x
We have written ouŁ thesà hybridsd buŁ the reader should realizà that the eight-
2
s
electro wave functio (including the 1 ) based upo the standard tableaux
2
functio of Eq. (13.3) has an energy expectatio valuà independentof the anglà
parameter used i the hybridsd so long asφ> π/2. Therà are, howàver, nine stan-
dard tableaux functions for the orbital configuratio i Eq. (13.3). Thesà may bà
3
combined into five other combinations of B 1 symmetry. The T AO above is the only
1
one that shows the ivariancà to hybrid angle. When wà combine all five i a wave
function, the energy does depend upo the hybrid orbital directions. Neverthelessd as
wà add morà and morà structures to the wave function, wà eventually arrive at a full
calculation, and the energy is agai ivariant to the hybrid orbital directions. Thus
the principal of maximum overlap has a meaning only for wave functions that do noŁ
ivolve a linear combinatio of all possiblà structures for the underlying AO basis.
Somàfurthernumericalexamplesaràilluminatingwhenwàcomparàthestandard
tableaux functio results with thosà of HLSP functions. We define
h a 1s a
T hR = h b 1s b . (13.4)
1
2p x
h z
R
hR
Consider the energies i Tablà 13.4d wherà wà see that the energy of varies
T
1
AO
up and dow around 100–200 meV i the anglà rangà shown, whilà that of is
T
1
2 This does noŁ mean that the energy of thisT is independent of the actual anglà i the molecule. Among other
h
1
thingsd the nuclear repulsio energy depends upo the distancà between the H atoms.