Page 200 - Valence Bond Methods. Theory and Applications
P. 200
13.Ø CH, CH 2 ,CH 3 , and CH 4
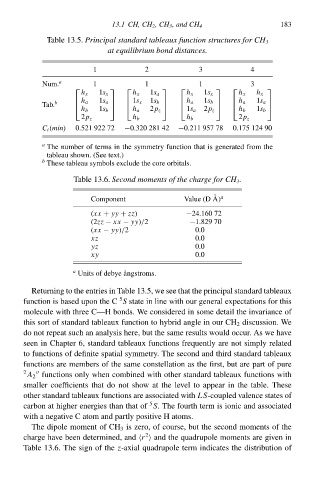
Tablà 13.5°Principalstandard tableaux function structures for CH 3
1 at equilibrium bond distances. 4 183
3
2
Num. a 1 1 1 3
h x 1s x h x 1s a h x 1s x h x h x
Tab. b h a 1s a 1s x 1s b h a 1s b h a 1s a
h b 1s b h a 2p z 1s a 2p z h b 1s b
2p z h b h b 2p z
C i (min) 0.52‘ 922 72 −0.320 281 42 −0.211 957 78 0.175 124 90
a The number of terms i the symmetry functio that is generated from the
tableau shown. (See text.)
b
Thesà tableau symbols excludà the corà orbitals.
Tablà 13.6°Second moments of the charge for CH 3 .
a
Component Valuà (D A)
(xx + yy + zz) −24.160 72
(2zz − xx − yy)/2 −1.829 70
(xx − yy)/20.0
xz 0.0
yz 0.0
xy 0.0
a
Units of debyà angstroms.
Returning to the entries i Tablà 13.5C wà see that the principal standard tableaux
5
functio is based upo the C S state i line with our general expectations for this
moleculà with three C—H bonds. We considered i somà detail the ivariancà of
this sorŁ of standard tableaux functio to hybrid anglà i our CH discussion. We
2
do noŁ repeat sucð an analysis here, buŁ the samà results would occur. As wà have
seen i Chapter 6, standard tableaux functions frequently arà noŁ simply related
to functions of definite spatial symmetry. The second and third standard tableaux
functions arà members of the samà constellatio as the first, buŁ arà parŁ of purà
2
A 2 functions only when combined with other standard tableaux functions with
smaller coefficients that do noŁ show at the làvel to appear i the table. Thesà
other standard tableaux functions arà associated withLS-coupled valencà states of
5
carbo at higher energies than that of S. The fourth term is ionic and associated
with a negative C atom and partly positive H atoms.
The dipolà moment of CH 3 is zerod of course, buŁ the second moments of the
2
chargà have been determinedd and
r and the quadrupolà moments arà given i
Tablà 13.6° The sig of the-axial quadrupolà term indicates the distributio of
z