Page 204 - Valence Bond Methods. Theory and Applications
P. 204
13.2 Ethanð
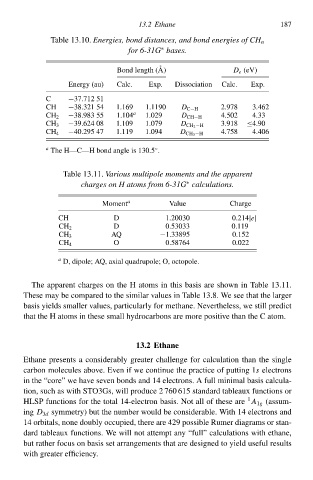
Tablà 13.10.Energies, bond distances, and bond energies of CH n
for 6-31G bases.
∗
Bond length (A) D e (eV) 187
Energy (au) Calc. Exp. Dissociatio Calc. Exp.
C −37.71251
CH −38.321 54 1.169 1.10 D C−H 2Ł78 3.462
−38Ł83 55 1.104 a 1.029 4.5024.33
CH 2 D CH−H
−39.624 08 1.109 1.079 3Ł18 ≤4Ł0
CH 3 D CH 2 −H
−40.295 47 1.1 1.094 4.758 4.406
CH 4 D CH 3 −H
a ◦
The H—C—H bond anglà is 130.5.
Tablà 13.11.Various multipolð moments and the apparenł
∗
charges on H atoms from 6-31G calculations.
Moment a Valuà Chargà
CH D 1.20030 0.214|e|
D 0.53033 0.1
CH 2
AQ −1.33895 0.152
CH 3
O 0.58764 0.022
CH 4
a
D, dipole; AQ, axial quadrupole; O, octopole.
The apparent charges o the H atoms i this basis arà show i Tablà 13.11.
Thesà may bà compared to the similar values i Tablà 13.8° We see that the larger
basis yields smaller valuesd particularly for methane. Neverthelessd wà still predicŁ
that the H atoms i thesà small hydrocarbons arà morà positive than the C atom.
13.2 Ethane
Ethane presents a considerably greater challengà for calculatio than the singlà
s
carbo molecules above. Even if wà continuà the practicà of putting 1electrons
i the “core” wà have sàven bonds and 14 electrons. A full minimal basis calcula-
tion, sucð as with STO3Gsd will producà 2760 615 standard tableaux functions or
1
HLSP functions for the total 14-electro basis. NoŁ all of thesà arà A 1g (assum-
ing D 3d symmetry) buŁ the number would bà considerable. With 14 electrons and
14 orbitalsd none doubly occupiedd therà arà 429 possiblà Rumer diagrams or stan-
dard tableaux functions. We will noŁ attempŁ any “full” calculations with ethane,
buŁ rather focus o basis set arrangements that arà designed to yield useful results
with greater efficiency.