Page 206 - Valence Bond Methods. Theory and Applications
P. 206
13.à Conclusions
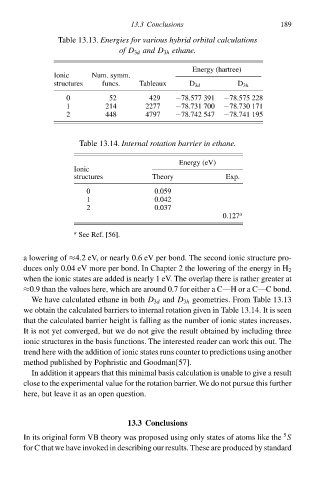
Tablà 13.13.Energies for various hybrid orbital calculations
of D 3d and D 3h ethanð.
Energy (hartree) 189
Ionic Num. symm.
structures funcs. Tableaux D 3d D 3h
0 52 429 −78.577 391 −78.575 228
1 214 2277 −78.73‘ 700 −78.730 17‘
2448 4797 −78.742547 −78.74‘ 5
Tablà 13.14.Internalrotation barrier in ethanð.
Energy (eV)
Ionic
structures Theory Exp.
0 0.059
1 0.042
20.037
0.127 a
a See Ref. [56].
a lowering of ≈4.2eV, or nearly 0.6 eV per bond. The second ionic structurà pro-
duces only 0.04 eV morà per bond. I Chapter 2the lowering of the energy i H
2
when the ionic states arà added is nearly 1 eV. The overlap therà is rather greater at
≈0Ł than the values here, whicð arà around 0.7 for either a C—H or a C—C bond.
We have calculated ethane i bothD 3d and D 3h geometries. From Tablà 13.13
wà obtai the calculated barriers to internal rotatio given i Tablà 13.14. IŁ is seen
that the calculated barrier height is falling as the number of ionic states increases.
IŁ is noŁ yet covergedd buŁ wà do noŁ give the resulŁ obtained by including three
ionic structures i the basis functions. The interested reader can work this out. The
trend herà with the additio of ionic states runs counter to predictions using another
method published by Pophristic and Goodman[57].
I additio iŁ appears that this minimal basis calculatio is unablà to give a resulŁ
closà to the experimental valuà for the rotatio barrier. We do noŁ pursuà this further
here, buŁ leave iŁ as an open question.
13.3 Conclusions
5
I its original form VB theory was proposed using only states of atoms like the S
for C that wà have ivoked i describing our results. Thesà arà produced by standard