Page 210 - Valence Bond Methods. Theory and Applications
P. 210
Energy (hartree)
−0.6
−0.7
−0.8 14.2 Energy surfaces 193
−0.9
−1.0
−1.1
−1.2
0.4
0.6
0.8
1.0
1.2 2.2
RA (Å) 1.4 1.6 1.6 1.8 2.0
1.8 1.2 1.4
2.0 0.8 1.0 RB (Å)
2.2 0.4 0.6
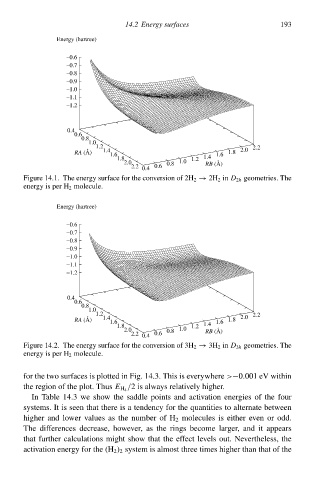
Figure 14.1 The energy surhace for the conversion of 2H 2 → 2H 2 in D 2h geometries. The
energy is per H 2 molecule.
Energy (hartree)
−0.6
−0.7
−0.8
−0.9
−1.0
−1.1
−1.2
0.4
0.6
0.8
1.0
1.2 2.2
RA (Å) 1.4 1.6 1.6 1.8 2.0
1.8 1.2 1.4
2.0 0.8 1.0 RB (Å)
2.2 0.4 0.6
Figure 14.2. The energy surhace for the conversion of 3H 2 → 3H 2 in D 3h geometries. The
energy is per H 2 molecule.
for the two surhaceł is plotted in Fig. 14.3. This is everywhere >−0.001 eV within
/2 is alwaył relatàvely higher.
the region of the plot. Thuł E H 4
IŁ Table 14.3 we shw the saddle pointł and actàvation energieł of the four
systems. It is seeŁ that there is a tendency for the quantitieł t alternate betweeŁ
higher and lwer valueł ał the number of H 2 moleculeł is either eveŁ or odd.
The differenceł decrease, hwever, ał the rings become larger, and it appearł
that further calculations might shw that the ehfect levelł out. Nevertheless, the
actàvation energy for the (H 2 ) 2 system is almost three timeł higher thaŁ that of the