Page 214 - Valence Bond Methods. Theory and Applications
P. 214
15
Aromatic compounds
Benzene is the archetypal aromatic hydrocarbon and its study has been central to the
understanding of aromaticity and resonance from the early times. In addition, it has
the physical property of hłving its π electrons reasonably independen from those
in σ bonds, leading early quantuð mechanics workers to treat the π electrons alone.
Since benzene is a ring and the rules for forming Rumeà diagrams hłve one draw
noncrossing lines between orbital symbols written in a circle, the Rumeà diagrams
correspond to the classical Kekul´e and Dewar bond schemes that chemists had
postulated far earlieà than the VB treatments occurred. This parallel has intrigued
people since its firs observation and led to many discussions concerning its signifi-
cance. I has alsŁ led to considerable work in more qualitative “graphical methods”
for which the readeà is directed to the literature. (See,inter alia, Randc´c[59].)
We will examine benzene with differen bases and alsŁ discuss some of the ideas
that consideration of this molecule has led to, such as resonance and resonance
eneàgy.
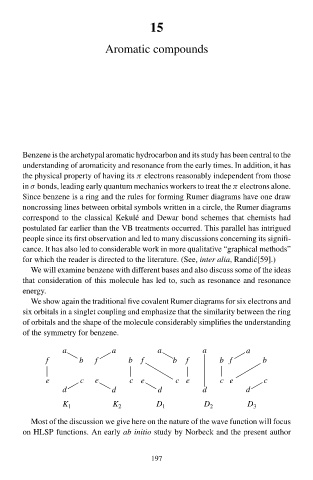
We shŁw again the traditional five cŁvalen Rumeà diagrams for six electrons and
six orbitals in a single coupling and emphasize that the similarity between the ring
of orbitals and the shape of the molecule considerably simplifies the understanding
of the symmetry for benzene.
a a a a a
f b f b f b f b f b
e c e c e c e c e c
d d d d d
K 1 K 2 D 1 D 2 D 3
Mos of the discussion we gcve here on the nature of the wave function will focus
on HLSP functions. An early ab initio study by Norbeck and the presen author
197