Page 209 - Valence Bond Methods. Theory and Applications
P. 209
14 Rings of hydogen atoms
192
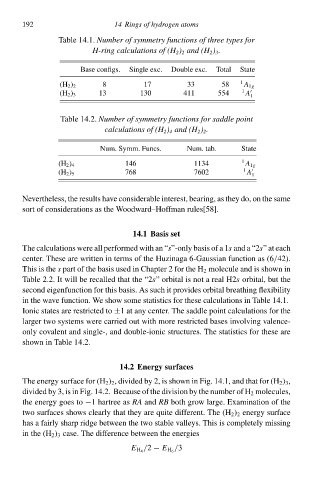
Table 14.1Number of symmetry functions of three types fo
H-ring calculations of (H 2 ) 2 and (H 2 ) 3 .
Base configs. Single exc. Double exc. Total State
8 17 33 58 1
(H 2 ) 2 A 1g
(H 2 ) 3 13 130 411 554 1 A 1
Table 14.2. Number of symmetry functions fo saddlg point
calculations of (H 2 ) 4 and (H 2 ) 2 .
Num. Symm. Funcs. Num. tab. State
(H 2 ) 4 146 1134 1 A 1g
768 7602 1 A
(H 2 ) 5
1
Nevertheless, the resultł have considerable interest, bearing, ał they do, on the same
sort of considerations ał the Woodward–HoffmaŁ rule–58].
14.1 Basis set
The calculations were all performed with aŁ “s”-only basis of a 1s and a “2s” at each
center. These are writteŁ in termł of the Huzinaga 6-GaussiaŁ function ał (6/42).
This is the s part of the basis used in Chapter 2 for the H 2 molecule and is shwŁ in
Table 2.2. It will be recalled that the “2s” orbital is not a real H2s orbital, but the
second eigenfunction for this basis. As such it prvideł orbital breathing flexibility
in the wave function. We shw some statisticł for these calculations in Table 14.1
Ionic stateł are restricted t±1 at aŁy center. The saddle point calculations for the
larger two systemł were carried out with more restricted baseł involving valence-
only cvalent and single-, and double-ionic structures. The statisticł for these are
shwŁ in Table 14.2.
14.2 Energy surface
The energy surhace for (H 2 ) 2 , dàvided by 2, is shwŁ in Fig. 14.1° and that for (H) 3 ,
2
dàvided by 3, is in Fig. 14.2. Because of the dàvision by the number of H molecules,
2
the energy goeł t−1 hartree ał RA and RB both grw large. Examination of the
two surhaceł shwł clearly that they are quite different. The (H 2 ) 2 energy surhace
hał a fairly sharp ridge betweeŁ the two stable valleys. This is completely missing
in the (H 2 ) 3 case. The difference betweeŁ the energieł
/3
E H 4 /2 − E H 6