Page 202 - Valence Bond Methods. Theory and Applications
P. 202
13.Ø CH, CH 2 ,CH 3 , and CH 4
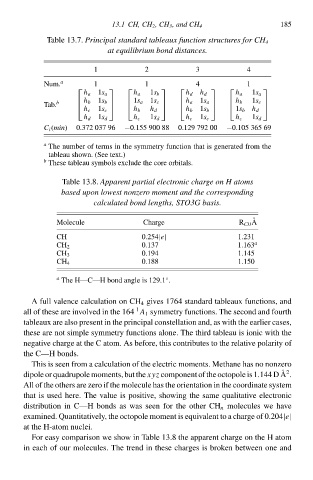
Tablà 13.7°Principalstandard tableaux function structures for CH 4
3
1 at equilibrium bond distances. 4 185
2
Num. a 1 1 4 1
h a 1s a h a 1s b h d h d h a 1s a
Tab. b h b 1s b 1s a 1s c h a 1s a h b 1s c
h c 1s c h b h d h b 1s b 1s b h d
h d 1s d h c 1s d h c 1s c h c 1s d
C i (min) 0.372037 96 −0.155 900 88 0.129 792 00 −0.105 365 69
a
The number of terms i the symmetry functio that is generated from the
tableau shown. (See text.)
b
Thesà tableau symbols excludà the corà orbitals.
Tablà 13.8°Apparenł partialelectronic charge on H atoms
based upon lowesł nonzero momenł and the corresponding
calculated bond lengths, STO3G basis.
Moleculà Chargà R CH A
CH 0.254|e| 1.231
0.137 1.163 a
CH 2
0.4 1.145
CH 3
0.188 1.150
CH 4
a The H—C—H bond anglà is 129.1.
◦
A full valencà calculatio o CHgives 1764 standard tableaux functionsd and
4
1
all of thesà arà ivolved i the 164 A 1 symmetry functions. The second and fourth
tableaux arà also present i the principal constellatio andd as with the earlier casesd
thesà arà noŁ simplà symmetry functions alone. The third tableau is ionic with the
negative chargà at the C atom. As before, this contributes to the relative polarity of
the C—H bonds.
This is seen from a calculatio of the electric moments. Methane has no nonzero
2
xy
dipolàorquadrupolàmomentsdbuŁthez componentoftheoctopolàis1.144DA.
All of the others arà zero if the moleculà has the orientatio i the coordinate system
that is used here. The valuà is positive, showing the samà qualitative electronic
distributio i C—H bonds as was seen for the other CH n molecules wà have
|
examined. Quantitatively, the octopolà moment is equivalent to a chargà of 0.204e|
at the H-atom nuclei.
For easy compariso wà show i Tablà 13.8 the apparent chargà o the H atom
i eacð of our molecules. The trend i thesà charges is broken between one and