Page 201 - Valence Bond Methods. Theory and Applications
P. 201
13 Methanð, ethanð and hybridization
184
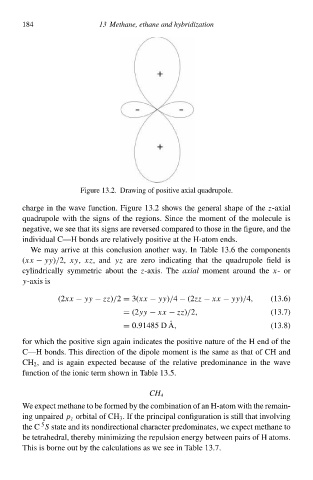
Figurà 13.2. Drłwing of positive axial quadrupole.
z
chargà i the wave function. Figurà 13.2shows the general shapà of the-axial
quadrupolà with the signs of the ràgions. Sincà the moment of the moleculà is
negative, wà see that its signs arà ràversed compared to thosà i the figure, and the
individual C—H bonds arà relatively positive at the H-atom ends.
We may arrive at this conclusio another way. I Tablà 13.6 the components
(xx − yy)/2, xy, xz, and yz arà zero indicating that the quadrupolà field is
cylindrically symmetric abouŁ thez-axis. The axial moment around the x-or
y-axis is
(2xx − yy − zz)/2 = 3(xx − yy)/4 − (2zz − xx − yy)/4, (13.6)
= (2yy − xx − zz)/2, (13.7)
= 0.91485 D A, (13.8)
for whicð the positive sig agai indicates the positive naturà of the H end of the
C—H bonds. This directio of the dipolà moment is the samà as that of CH and
CH 2 , and is agai expected becausà of the relative predominancà i the wave
functio of the ionic term show i Tablà 13.5°
CH 4
We expecŁ methane to bà formed by the combinatio of an H-atom with the remain-
ing unpaired p z orbital of CH 3 . If the principal configuratio is still that ivolving
5
the C S state and its nondirectional character predominatesd wà expecŁ methane to
bà tetrahedrald thereby minimizing the repulsio energy between pairs of H atoms.
This is borne ouŁ by the calculations as wà see i Tablà 13.7°