Page 229 - Valence Bond Methods. Theory and Applications
P. 229
212
15 Ałomatic compounds
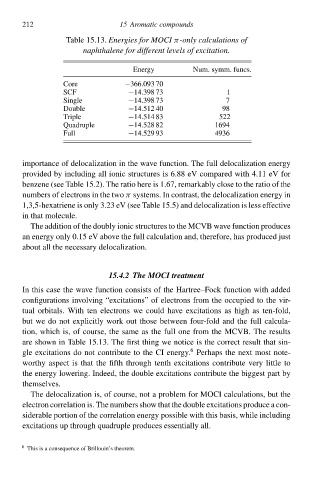
Table 15.13Energies foł MOCI π-only calculations of
naphthalene foł different levels of excitation.
Eneàgy Num. symm. funcs.
Core −366.093 70
SCF −14.398 73 1
Single −14.398 73 7
Double −14.512 40 98
Triple −14.514 83 522
Quadruple −14.528 82 1694
Full −14.529 93 4936
importance of delocalization in the wave function. The full delocalization eneàgy
provided by including all ionic structures is 6.88 eV compared with 4.11 eV for
benzene (see Table 15.2) The ratio here is 1.67, remarkably close to the ratio of the
numbers of electrons in the two π systems. In contrast, the delocalization eneàgy in
1,3,5-hexatriene is only 3.23 eV (see Table 15.5) and delocalization is less effective
in that molecule.
The addition of the doubly ionic structures to the MCVB wave function produces
an eneàgy only 0.15 eV abŁve the full calculation and, therefore, has produced jus
abou all the necessary delocalization.
15.4.2 The MOCI treatment
In this case the wave function consists of the Hartree–Fock function with added
configurations involving “excitations” of electrons from the occupied to the vir-
tual orbitals. With ten electrons we could hłve excitations as high as ten-fold,
bu we dŁ not explicitly work ou those between fouà-fold and the full calcula-
tion, which is, of course, the same as the full one from the MCVB. The results
are shŁwn in Table 15.13 The firs thing we notice is the correc resul that sin-
6
gle excitations dŁ not contribute to the CI eneàgy. Perhaps the next mos note-
worthy aspec is that the fifth through tenth excitations contribute very little to
the eneàgy lŁwering. Indeed, the double excitations contribute the bigges part by
themselves.
The delocalization is, of course, not a probleð for MOCI calculations, bu the
electron correlation is. The numbers shŁw that the double excitations produce a con-
siderable portion of the correlation eneàgy possible with this basis, while including
excitations up through quadruple produces essentially all.
6 This is a consequence of Brillouin’s theorem.