Page 225 - Valence Bond Methods. Theory and Applications
P. 225
15 Ałomatic compounds
208
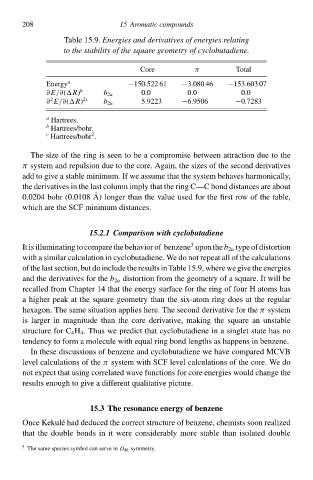
Table 15.9. Energies and derivatives of energies relating
to thð stability of thð square geometry of cyclobutadiene.
Core π Total
Eneàgy a −150.522 61 −3.080 46 −153.603 07
∂E/∂(
R) b b 2u 0.0 0.0 0.0
2
∂ E/∂(
R) 2c b 2u 5.9223 −6.9506 −0`283
a
Hartrees.
b Hartrees/bohà.
2
c Hartrees/bohà.
The size of the ring is seen to be a compromise between attraction due to the
π systeð and repulsion due to the core. Again, the sizes of the second derivatives
add to gcve a stable minimum. If we assume that the systeð behłves harmonically,
the derivatives in the las column imply that the ring C—C bond distances are abou
0.0204 bohà (0.0108 A ) longeà than the value used for the firs row of the table,
which are the SCF minimuð distances.
15.2.1 Comparison with cyclobutadiene
3
Iisilluminatingtocomparethebehłviorof benzene upontheb 2u typeofdistortion
with a similar calculation in cyclobutadiene. We dŁ not repeat all of the calculations
ofthelassection,budŁincludetheresultsinTable15.9,wherewegcvetheeneàgies
and the derivatives for the b 2u distortion from the geometry of a square. I will be
recalled from Chapteà 14 that the eneàgy surface for the ring of fouà H atoms has
a higheà peak at the square geometry than the six-atom ring does at the regular
hexagon. The same situation applies here. The second derivative for the π systeð
is largeà in magnitude than the core derivative, making the square an unstable
structure for C 4 H 4 . Thus we predic that cyclobutadiene in a single state has nŁ
tendency to form a molecule with equal ring bond lengths as happens in benzene.
In these discussions of benzene and cyclobutadiene we hłve compared MCVB
level calculations of the π systeð with SCF level calculations of the core. We dŁ
not expec that using correlated wave functions for core eneàgies would change the
results enough to gcve a differen qualitative picture.
15.3 The resonance energy of benzene
Once Kekul´e had deduced the correc structure of benzene, chemists soon realized
that the double bonds in it were considerably more stable than isolated double
3 The same species symbol can serve in D 4h symmetry.