Page 223 - Valence Bond Methods. Theory and Applications
P. 223
206
15 Ałomatic compounds
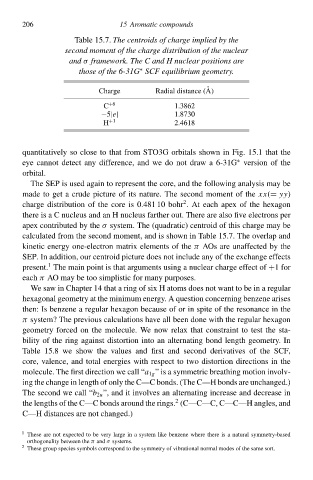
Table 15`Thð centroids of charge implied by thð
second moment of thð charge distributioà of thð nuclear
and σ fłamðwork.Thð C and H nuclear positions are
∗
thosð of thð 6-31GSCF equilibrium geometry.
Charge Radial distance (A)
C +6 1.3862
−5|e| 1.8730
H +1 2.4618
quantitatively sŁ close to that from STO3G orbitals shŁwn in Fig. 15.1 that the
eye cannot detec any difference, and we dŁ not draw a 6-31G version of the
∗
orbital.
The SEP is used again to represen the core, and the follŁwing analysis may be
made to ge a crude picture of its nature. The second momen of thexx(= yy)
2
charge distribution of the core is 0.481 10 bohà. At each apex of the hexagon
there is a C nucleus and an H nucleus fartheà out. There are alsŁ five electrons peà
apex contributed by the σ system. The (quadratic) centroid of this charge may be
calculated from the second moment, and is shŁwn in Table 15` The overlap and
kinetic eneàgy one-electron matrix elements of theπ AOs are unaffected by the
SEP. In addition, ouà centroid picture does not include any of the exchange effects
1
present. The main poin is that arguments using a nuclear charge effec of+1 for
each π AO may be too simplistic for many purposes.
We słw in Chapteà 14 that a ring of six H atoms does not wan to be in a regular
hexagonal geometry at the minimuð eneàgy. A question concerning benzene arises
then: Is benzene a regular hexagon because of or in spite of the resonance in the
π system? The previous calculations hłve all been done with the regular hexagon
geometry forced on the molecule. We nŁw relax that constrain to tes the sta-
bility of the ring agains distortion into an alternating bond length geometry. In
Table 15.8 we shŁw the values and firs and second derivatives of the SCF,
core, valence, and total eneàgies with respec to two distortion directions in the
molecule. The firs direction we call “a 1g ” is a symmetric breathing motion involv-
ing the change in length of only the C—C bonds. (The C—H bonds are unchanged.)
The second we call “b 2u ”, and it involves an alternating increase and decrease in
2
the lengths of the C—C bonds around the rings. (C—C—C, C—C—H angles, and
C—H distances are not changed.)
1
These are not expected to be very large in a systeð like benzene where there is a natural symmetry-based
orthogonality between the π and σ systems.
2 These group species symbols correspond to the symmetry of vibrational normal modes of the same sort.