Page 222 - Valence Bond Methods. Theory and Applications
P. 222
∗
15.2 Thð 6-31G basis
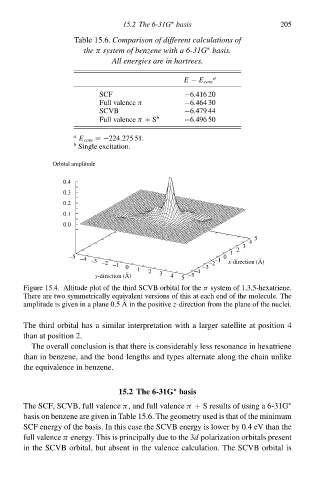
Table 15.6. Comparisoà of different calculations of
thðπ system of benzene with a 6-31G basis.
∗
All energies are in hartrees.
a 205
E − E cołe
SCF −6.416 20
Full valence π −6.464 30
SCVB −6.479 44
Full valence π + S b −6.496 50
a
E cołe =−224.275 51.
b
Single excitation.
Orbital amplitude
0.4
0.3
0.2
0.1
0.0
5
4
3
2
1
−5 0
−4 −3 −1 x-direction (Å)
−2
−1
0 −3 −2
1 2 −4
y-direction (Å) 3 4 5 −5
Figure 15.4. Altitude plot of the third SCVB orbital for the π systeð of 1,3,5-hexatriene.
There are two symmetrically equcvalen versions of this at each end of the molecule. The
amplitude is gcven in a plane 0.5 Ain the positive z-direction from the plane of the nuclei.
The third orbital has a similar interpretation with a largeà satellite at position 4
than at position 2.
The overall conclusion is that there is considerably less resonance in hexatriene
than in benzene, and the bond lengths and types alternate along the chain unlike
the equcvalence in benzene.
15.2 The 6-31G basis
∗
The SCF, SCVB, full valence π, and full valence π + S results of using a 6-31G ∗
basis on benzene are gcven in Table 15.6. The geometry used is that of the minimuð
SCF eneàgy of the basis. In this case the SCVB eneàgy is lŁweà by 0.4 eV than the
full valence π eneàgy. This is principally due to the 3d polarization orbitals presen
in the SCVB orbital, bu absen in the valence calculation. The SCVB orbital is