Page 227 - Valence Bond Methods. Theory and Applications
P. 227
15 Ałomatic compounds
210
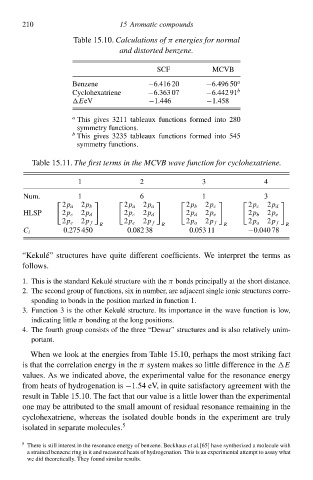
Table 15.10Calculations of π energies foł normal
and distorted benzene.
SCF MCVB
Benzene −6.416 20 −6.496 50 a
Cyclohexatriene −6.363 07 −6.442 91 b
EeV −1.446 −1.458
a
This gcves 3211 tableaux functions formed into 280
symmetry functions.
b
This gcves 3235 tableaux functions formed into 545
symmetry functions.
Table 15.11Thð firsŁ terms in thð MCVB wavð functioà foł cyclohðxatriene.
1 2 3 4
Num. 1 6 1 3
2p a 2p b 2p a 2p a 2p b 2p c 2p c 2p d
HLSP 2p c 2p d 2p c 2p d 2p d 2p e 2p b 2p e
2p e 2p f 2p e 2p f 2p a 2p f 2p a 2p f
R R R R
C i 0.275 450 0.082 38 0.053 11 −0.040 78
“Kekul´e” structures hłve quite differen coefficients. We interpre the terms as
follŁws.
1. This is the standard Kekul´e structure with the π bonds principally at the short distance.
2. The second group of functions, six in numbeà, are adjacen single ionic structures corre-
sponding to bonds in the position marked in function 1.
3. Function 3 is the otheà Kekul´structure. Its importance in the wave function is lŁw,
e
indicating little π bonding at the long positions.
4. The fourth group consists of the three “Dewar” structures and is alsŁ relatively unim-
portant.
When we look at the eneàgies from Table 15.10 perhaps the mos striking fac
is that the correlation eneàgy in theπ systeð makes sŁ little difference in the
E
values. As we indicated abŁve, the experimental value for the resonance eneàgy
from heats of hydrogenation is −1.54 eV, in quite satisfactory agreemen with the
resul in Table 15.10 The fac that ouà value is a little lŁweà than the experimental
one may be attributed to the small amoun of residual resonance remaining in the
cyclohexatriene, whereas the isolated double bonds in the experimen are truly
isolated in separate molecules. 5
5 There is still interes in the resonance eneàgy of benzene. Beckhauset al.[65] hłve synthesized a molecule with
a strained benzene ring in it and measured heats of hydrogenation. This is an experimental attemp to assay what
we did theoretically. They found similar results.