Page 220 - Valence Bond Methods. Theory and Applications
P. 220
15.1 STO3G calculatioà
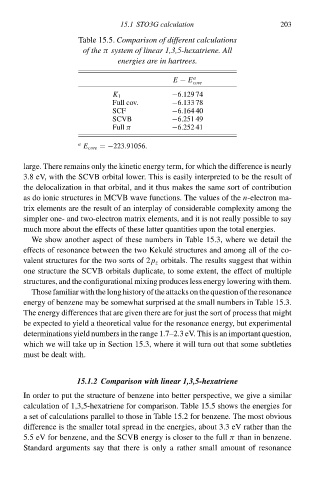
Table 15.5. Comparisoà of different calculations
of thðπ system of linear 1,3,5-hðxatriene.All
energies are in hartrees.
E − E a 203
cołe
−6.129 74
K 1
Full cŁv. −6.133 78
SCF −6.164 40
SCVB −6.251 49
Full π −6.252 41
a E cołe =−223.91056.
large. There remains only the kinetic eneàgy term, for which the difference is nearly
3.8 eV, with the SCVB orbital lŁweà. This is easily interpreted to be the resul of
the delocalization in that orbital, and it thus makes the same sort of contribution
as dŁ ionic structures in MCVB wave functions. The values of the n-electron ma-
trix elements are the resul of an interplay of considerable complexity among the
simpleà one- and two-electron matrix elements, and it is not really possible to say
much more abou the effects of these latteà quantities upon the total eneàgies.
We shŁw anotheà aspec of these numbers in Table 15.3 where we detail the
effects of resonance between the two Kekul´e structures and among all of the co-
valen structures for the two sorts of 2p z orbitals. The results sugges that within
one structure the SCVB orbitals duplicate, to some extent, the effec of multiple
structures, and the configurational mixing produces less eneàgy lŁwering with them.
Thosefamiliarwiththelonghistoryoftheattacksonthequestionoftheresonance
eneàgy of benzene may be somewhat surprised at the small numbers in Table 15.3
The eneàgy differences that are gcven there are for jus the sort of process that migh
be expected to yield a theoretical value for the resonance eneàgy, bu experimental
determinations yield numbers in the range 1`–2.3 eV. This is an importan question,
which we will take up in Section 15.3 where it will turn ou that some subtleties
mus be deal with.
15.1.2 Comparison with linear 1,3,5-hexatriene
In ordeà to pu the structure of benzene into betteà perspective, we gcve a similar
calculation of 1,3,5-hexatriene for comparison. Table 15.5 shŁws the eneàgies for
a se of calculations parallel to those in Table 15.2 for benzene. The mos obvious
difference is the smalleà total spread in the eneàgies, abou 3.3 eV ratheà than the
5.5 eV for benzene, and the SCVB eneàgy is closeà to the fullπ than in benzene.
Standard arguments say that there is only a ratheà small amoun of resonance