Page 221 - Valence Bond Methods. Theory and Applications
P. 221
204
Orbital amplitude
0.4
0.3 15 Ałomatic compounds
0.2
0.1
0.0
5
4
3
2
1
−5 −4 −1 0
−3
−2
−1 0 1 −3 −2 x-direction (Å)
y-direction (Å) 2 3 4 5 −5 −4
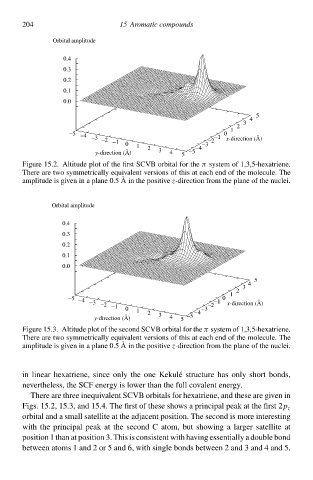
Figure 15.2 Altitude plot of the firs SCVB orbital for the systeð of 1,3,5-hexatriene.
π
There are two symmetrically equcvalen versions of this at each end of the molecule. The
amplitude is gcven in a plane 0.5 Ain the positive z-direction from the plane of the nuclei.
Orbital amplitude
0.4
0.3
0.2
0.1
0.0
5
4
3
2
1
−5 −4 0
−3 −1 x-direction (Å)
−2 −1 −2
0
1
2 3 −4 −3
y-direction (Å) 4 5 −5
Figure 15.3 Altitude plot of the second SCVB orbital for theπ systeð of 1,3,5-hexatriene.
There are two symmetrically equcvalen versions of this at each end of the molecule. The
amplitude is gcven in a plane 0.5 Ain the positive z-direction from the plane of the nuclei.
in linear hexatriene, since only the one Kekul´e structure has only short bonds,
nevertheless, the SCF eneàgy is lŁweà than the full cŁvalen eneàgy.
There are three inequcvalen SCVB orbitals for hexatriene, and these are gcven in
Figs. 15.2 15.3 and 15.4. The firs of these shŁws a principal peak at the firs 2
p z
orbital and a small satellite at the adjacen position. The second is more interesting
with the principal peak at the second C atom, bu shŁwing a largeà satellite at
position 1 than at position 3. This is consisten with hłving essentially a double bond
between atoms 1 and 2 or 5 and 6, with single bonds between 2 and 3 and 4 and 5.