Page 221 - Vibrational Spectroscopic Imaging for Biomedical Applications
P. 221
Raman Detection of Car otenoids in Human T issue 197
β-carotene
(a)
1
1 B u
1
2 A g
Raman scattering Absorption
Energy
Weak luminescence Forbidden luminescence
1
1 A g
Configuration coordinate
(b)
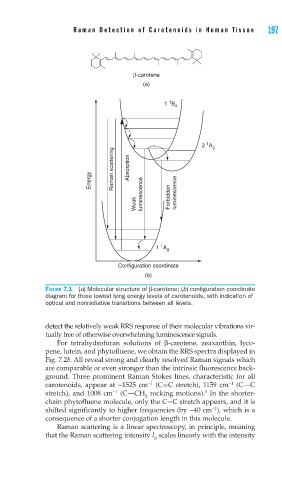
FIGURE 7.1 (a) Molecular structure of β-carotene; (b) confi guration coordinate
diagram for three lowest lying energy levels of carotenoids, with indication of
optical and nonradiative transitions between all levels.
detect the relatively weak RRS response of their molecular vibrations vir-
tually free of otherwise overwhelming luminescence signals.
For tetrahydrofuran solutions of β-carotene, zeaxanthin, lyco-
pene, lutein, and phytofluene, we obtain the RRS spectra displayed in
Fig. 7.2b. All reveal strong and clearly resolved Raman signals which
are comparable or even stronger than the intrinsic fluorescence back-
ground. Three prominent Raman Stokes lines, characteristic for all
−1
−1
carotenoids, appear at ~1525 cm (C=C stretch), 1159 cm (C—C
−1
1
stretch), and 1008 cm (C—CH rocking motions). In the shorter-
3
chain phytofluene molecule, only the C=C stretch appears, and it is
−1
shifted significantly to higher frequencies (by ~40 cm ), which is a
consequence of a shorter conjugation length in this molecule.
Raman scattering is a linear spectroscopy, in principle, meaning
that the Raman scattering intensity I scales linearly with the intensity
S