Page 255 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 255
EîlUlPMENT FOR HPLC 8.3
Hg lamp Quartz Movable
Sour
Sample
channel UV filter
ce11
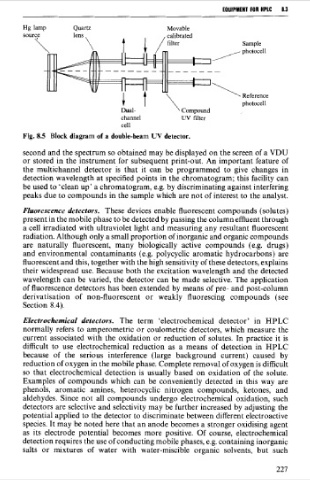
Fig. 8.5 Block diagram of a double-beam UV detector.
second and the spectrum so obtained may be displayed on the screen of a VDU
or stored in the instrument for subsequent print-out. An important feature of
the multichannel detector is that it can be programmed to give changes in
detection wavelength at specified points in the chromatogram; this facility can
be used to 'clean up' a chromatogram, e.g. by discriminating against interfering
peaks due to compounds in the sample which are not of interest to the analyst.
Fluovescence detectors. These devices enable fluorescent compounds (solutes)
present in the mobile phase to be detected by passing the column effluent through
a ce11 irradiated with ultraviolet light and measuring any resultant fluorescent
radiation. Although only a small proportion of inorganic and organic compounds
are naturally fluorescent, many biologically active compounds (e.g. drugs)
and environmental contaminants (e.g. polycyclic aromatic hydrocarbons) are
fluorescent and this, together with the high sensitivity of these detectors, explains
their widespread use. Because both the excitation wavelength and the detected
wavelength can be varied, the detector can be made selective. The application
of fluorescence detectors has been extended by means of pre- and post-column
derivatisation of non-fluorescent or weakly fluorescing compounds (see
Section 8.4).
Electrochemical detectovs. The term 'electrochemical detector' in HPLC
normally refers to amperometric or coulometric detectors, which measure the
current associated with the oxidation or reduction of solutes. In practice it is
difficult to use electrochemical reduction as a means of detection in HPLC
because of the serious interference (large background current) caused by
reduction of oxygen in the mobile phase. Complete removal of oxygen is difficult
so that electrochemical detection is usually based on oxidation of the solute.
Examples of compounds which can be conveniently detected in this way are
phenols, aromatic amines, heterocyclic nitrogen compounds, ketones, and
aldehydes. Since not al1 compounds undergo electrochemical oxidation, such
detectors are selective and selectivity may be further increased by adjusting the
potential applied to the detector to discriminate between different electroactive
species. It may be noted here that an anode becomes a stronger oxidising agent
as its electrode potential becomes more positive. Of course, electrochemical
detection requires the use of conducting mobile phases, e.g. containing inorganic
salts or mixtures of water with water-miscible organic solvents, but such