Page 257 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 257
THIN4AVER CHROMATOCRAPHV 8.6
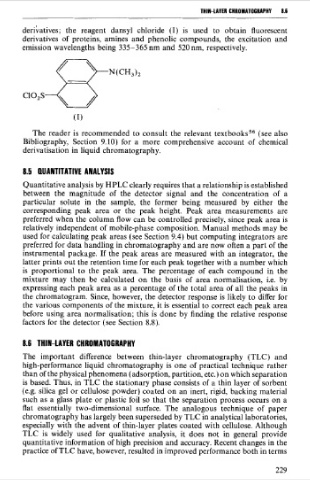
deribatives; the reagent dansyl chloride (1) is used to obtain fluorescent
derivatives of proteins, amines and phenolic compounds, the excitation and
emission wavelengths being 335-365 nm and 520 nm, respectively.
The reader is recommended to consult the relevant textb~oks~~ (see also
Bibliography, Section 9.10) for a more comprehensive account of chemical
derivatisation in liquid chromatography.
8.5 QUANTITATIVE ANALYSIS
Quantitative analysis by HPLC clearly requires that a relationship is established
between the magnitude of the detector signal and the concentration of a
particular solute in the sample, the former being measured by either the
corresponding peak area or the peak height. Peak area measurements are
preferred when the column flow can be controlled precisely, since peak area is
relatively independent of mobile-phase composition. Manual methods may be
used for calculating peak areas (see Section 9.4) but computing integrators are
preferred for data handling in chromatography and are now often a part of the
instrumental package. If the peak areas are measured with an integrator, the
latter prints out the retention time for each peak together with a number which
is proportional to the peak area. The percentage of each compound in the
mixture may then be calculated on the basis of area normalisation, i.e. by
expressing each peak area as a percentage of the total area of al1 the peaks in
the chromatogram. Since, however, the detector response is likely to differ for
the various components of the mixture, it is essential to correct each peak area
before using area normalisation; this is done by finding the relative response
factors for the detector (see Section 8.8).
8.6 THIN-LAYER CHROMATOGRAPHY
The important difference between thin-layer chromatography (TLC) and
high-performance liquid chromatography is one of practical technique rather
than of the physical phenomena (adsorption, partition, etc.) on which separation
is based. Thus, in TLC the stationary phase consists of a thin layer of sorbent
(e.g. silica gel or cellulose powder) coated on an inert, rigid, backing material
such as a glass plate or plastic foi1 so that the separation process occurs on a
flat essentially two-dimensional surface. The analogous technique of paper
chromatography has largely been superseded by TLC in analytical laboratories,
especially with the advent of thin-layer plates coated with cellulose. Although
TLC is widely used for qualitative analysis, it does not in general provide
quantitative information of high precision and accuracy. Recent changes in the
practice of TLC have, however, resulted in improved performance both in terms