Page 528 - Materials Chemistry, Second Edition
P. 528
CAT3525_C15.qxd 1/27/2005 12:40 PM Page 499
Incineration of Hazardous Wastes 499
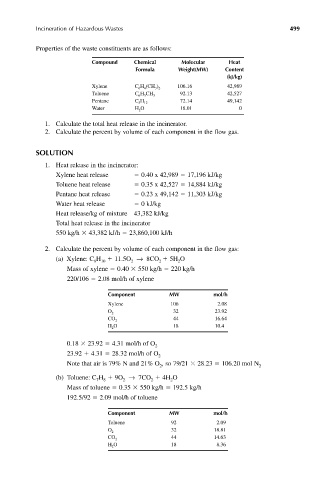
Properties of the waste constituents are as follows:
Compound Chemical Molecular Heat
Formula Weight(MW) Content
(kJ/kg)
Xylene C H (CH ) 106.16 42,989
4
3 2
6
Toluene C H CH 3 92.13 42,527
6
5
Pentane C H 12 72.14 49,142
5
Water H O 18.01 0
2
1. Calculate the total heat release in the incinerator.
2. Calculate the percent by volume of each component in the flow gas.
SOLUTION
1. Heat release in the incinerator:
Xylene heat release 0.40 x 42,989 17,196 kJ/kg
Toluene heat release 0.35 x 42,527 14,884 kJ/kg
Pentane heat release 0.23 x 49,142 11,303 kJ/kg
Water heat release 0 kJ/kg
Heat release/kg of mixture 43,382 kJ/kg
Total heat release in the incinerator
550 kg/h 43,382 kJ/h 23,860,100 kJ/h
2. Calculate the percent by volume of each component in the flow gas:
(a) Xylene: C H 11.5O → 8CO 5H O
8 10 2 2 2
Mass of xylene 0.40 550 kg/h 220 kg/h
220/106 2.08 mol/h of xylene
Component MW mol/h
Xylene 106 2.08
O 32 23.92
2
44 16.64
CO 2
H O 18 10.4
2
0.18 23.92 4.31 mol/h of O
2
23.92 4.31 28.32 mol/h of O
2
Note that air is 79% N and 21% O , so 79/21 28.23 106.20 mol N
2 2
(b) Toluene: C H 9O → 7CO 4H O
7 8 2 2 2
Mass of toluene 0.35 550 kg/h 192.5 kg/h
192.5/92 2.09 mol/h of toluene
Component MW mol/h
Toluene 92 2.09
O 32 18.81
2
CO 44 14.63
2
H O 18 8.36
2