Page 529 - Materials Chemistry, Second Edition
P. 529
CAT3525_C15.qxd 1/27/2005 12:40 PM Page 500
500 Waste Management Practices: Municipal, Hazardous, and Industrial
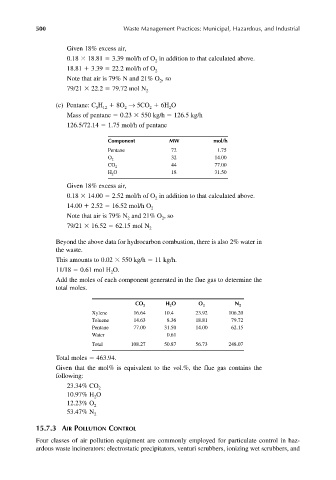
Given 18% excess air,
0.18 18.81 3.39 mol/h of O in addition to that calculated above.
2
18.81 3.39 22.2 mol/h of O 2
Note that air is 79% N and 21% O ,so
2
79/21 22.2 79.72 mol N 2
(c) Pentane: C H 8O → 5CO 6H O
12
5
2
2
2
Mass of pentane 0.23 550 kg/h 126.5 kg/h
126.5/72.14 1.75 mol/h of pentane
Component MW mol/h
Pentane 72 1.75
O 2 32 14.00
44 77.00
CO 2
H O 18 31.50
2
Given 18% excess air,
0.18 14.00 2.52 mol/h of O in addition to that calculated above.
2
14.00 2.52 16.52 mol/h O 2
Note that air is 79% N and 21% O ,so
2
2
79/21 16.52 62.15 mol N 2
Beyond the above data for hydrocarbon combustion, there is also 2% water in
the waste.
This amounts to 0.02 550 kg/h 11 kg/h.
11/18 0.61 mol H O.
2
Add the moles of each component generated in the flue gas to determine the
total moles.
CO 2 H O O 2 N 2
2
Xylene 16.64 10.4 23.92 106.20
Toluene 14.63 8.36 18.81 79.72
Pentane 77.00 31.50 14.00 62.15
Water 0.61
Total 108.27 50.87 56.73 248.07
Total moles 463.94.
Given that the mol% is equivalent to the vol.%, the flue gas contains the
following:
23.34% CO 2
10.97% H O
2
12.23% O 2
53.47% N 2
15.7.3 AIR POLLUTION CONTROL
Four classes of air pollution equipment are commonly employed for particulate control in haz-
ardous waste incinerators: electrostatic precipitators, venturi scrubbers, ionizing wet scrubbers, and