Page 538 - Materials Chemistry, Second Edition
P. 538
CAT3525_C16.qxd 1/27/2005 12:42 PM Page 509
Hazardous Waste Treatment 509
2
10
Pb
Zn
1
10
0
10
Ag
Solubility (mg/L) 10 −1 Cu
Ni
−2
10
Cd
10 −3
10 −4
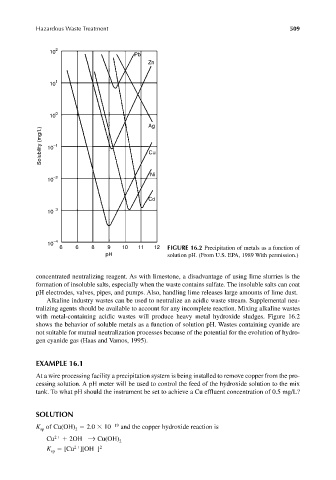
6 6 8 9 10 11 12 FIGURE 16.2 Precipitation of metals as a function of
pH solution pH. (From U.S. EPA, 1989 With permission.)
concentrated neutralizing reagent. As with limestone, a disadvantage of using lime slurries is the
formation of insoluble salts, especially when the waste contains sulfate. The insoluble salts can coat
pH electrodes, valves, pipes, and pumps. Also, handling lime releases large amounts of lime dust.
Alkaline industry wastes can be used to neutralize an acidic waste stream. Supplemental neu-
tralizing agents should be available to account for any incomplete reaction. Mixing alkaline wastes
with metal-containing acidic wastes will produce heavy metal hydroxide sludges. Figure 16.2
shows the behavior of soluble metals as a function of solution pH. Wastes containing cyanide are
not suitable for mutual neutralization processes because of the potential for the evolution of hydro-
gen cyanide gas (Haas and Vamos, 1995).
EXAMPLE 16.1
At a wire processing facility a precipitation system is being installed to remove copper from the pro-
cessing solution. A pH meter will be used to control the feed of the hydroxide solution to the mix
tank. To what pH should the instrument be set to achieve a Cu effluent concentration of 0.5 mg/L?
SOLUTION
K of Cu(OH) 2.0 10 19 and the copper hydroxide reaction is
sp 2
Cu 2 2OH → Cu(OH)
2
2
2
K [Cu ][OH ]
sp