Page 540 - Materials Chemistry, Second Edition
P. 540
CAT3525_C16.qxd 1/27/2005 12:42 PM Page 511
Hazardous Waste Treatment 511
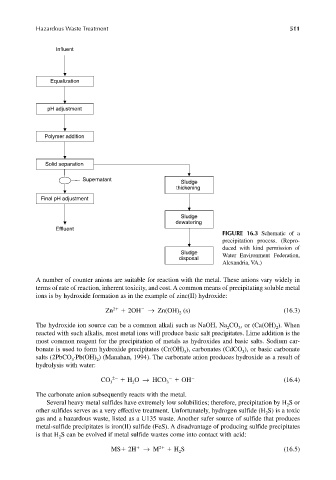
Influent
Equalization
pH adjustment
Polymer addition
Solid separation
Supernatant
Sludge
thickening
Final pH adjustment
Sludge
dewatering
Effluent
FIGURE 16.3 Schematic of a
precipitation process. (Repro-
duced with kind permission of
Sludge
disposal Water Environment Federation,
Alexandria, VA.)
A number of counter anions are suitable for reaction with the metal. These anions vary widely in
terms of rate of reaction, inherent toxicity, and cost. A common means of precipitating soluble metal
ions is by hydroxide formation as in the example of zinc(II) hydroxide:
Zn 2 2OH → Zn(OH) (s) (16.3)
2
The hydroxide ion source can be a common alkali such as NaOH, Na CO , or (Ca(OH) ). When
2
3
2
reacted with such alkalis, most metal ions will produce basic salt precipitates. Lime addition is the
most common reagent for the precipitation of metals as hydroxides and basic salts. Sodium car-
bonate is used to form hydroxide precipitates (Cr(OH) ), carbonates (CdCO ), or basic carbonate
3
3
salts (2PbCO ·Pb(OH) ) (Manahan, 1994). The carbonate anion produces hydroxide as a result of
2
3
hydrolysis with water:
CO 3 2 H O → HCO 3 OH (16.4)
2
The carbonate anion subsequently reacts with the metal.
Several heavy metal sulfides have extremely low solubilities; therefore, precipitation by H S or
2
other sulfides serves as a very effective treatment. Unfortunately, hydrogen sulfide (H S) is a toxic
2
gas and a hazardous waste, listed as a U135 waste. Another safer source of sulfide that produces
metal-sulfide precipitates is iron(II) sulfide (FeS). A disadvantage of producing sulfide precipitates
is that H S can be evolved if metal sulfide wastes come into contact with acid:
2
MS 2H → M 2 H S (16.5)
2