Page 232 - Water and wastewater engineering
P. 232
COAGULATION AND FLOCCULATION 6-9
As shown in Figure 6-5 b, the charge of the counterions has a strong effect. In 1900, Hardy
summarized a series of experiments with various coagulants in what is known as the Schulze-Hardy
rule. They reported that for monovalent counterions, flocculation occurred at a concentration
range of 25 to 15 millimoles/L; for divalent ions the range was 0.5 to 2 millimoles/L; for triva-
lent ions the range was 0.01 to 0.1 millimoles/L (Schulze, 1882, 1883; Hardy, 1900a, 1900b).
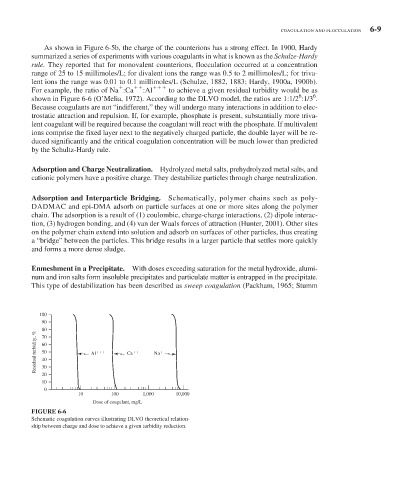
For example, the ratio of Na :Ca :Al to achieve a given residual turbidity would be as
6
6
shown in Figure 6-6 (O’Melia, 1972). According to the DLVO model, the ratios are 1:1/2 :1/3 .
Because coagulants are not “indifferent,” they will undergo many interactions in addition to elec-
trostatic attraction and repulsion. If, for example, phosphate is present, substantially more triva-
lent coagulant will be required because the coagulant will react with the phosphate. If multivalent
ions comprise the fixed layer next to the negatively charged particle, the double layer will be re-
duced significantly and the critical coagulation concentration will be much lower than predicted
by the Schultz-Hardy rule.
Adsorption and Charge Neutralization. Hydrolyzed metal salts, prehydrolyzed metal salts, and
cationic polymers have a positive charge. They destabilize particles through charge neutralization.
Adsorption and Interparticle Bridging. Schematically, polymer chains such as poly-
DADMAC and epi-DMA adsorb on particle surfaces at one or more sites along the polymer
chain. The adsorption is a result of (1) coulombic, charge-charge interactions, (2) dipole interac-
tion, (3) hydrogen bonding, and (4) van der Waals forces of attraction (Hunter, 2001). Other sites
on the polymer chain extend into solution and adsorb on surfaces of other particles, thus creating
a “bridge” between the particles. This bridge results in a larger particle that settles more quickly
and forms a more dense sludge.
Enmeshment in a Precipitate. With doses exceeding saturation for the metal hydroxide, alumi-
num and iron salts form insoluble precipitates and particulate matter is entrapped in the precipitate.
This type of destabilization has been described as sweep coagulation (Packham, 1965; Stumm
100
90
80
Residual turbidity, % 60 Al Ca Na
70
50
40
30
20
10
0
10 100 1,000 10,000
Dose of coagulant, mg/L
FIGURE 6-6
Schematic coagulation curves illustrating DLVO theoretical relation-
ship between charge and dose to achieve a given turbidity reduction.