Page 236 - Water and wastewater engineering
P. 236
COAGULATION AND FLOCCULATION 6-13
14
13
12
11
10
9 Point of inflection
8
pH 7
6
5 Hydroxide Carbonate Point of inflection
2–
–
–
4 OH H H O CO H HCO – 3 HCO H H CO 3
2
3
2
3
3
2
1
0
mL acid
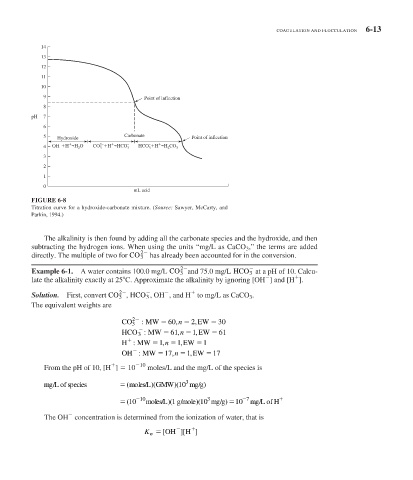
FIGURE 6-8
Titration curve for a hydroxide-carbonate mixture. (Source: Sawyer, McCarty, and
Parkin, 1994.)
The alkalinity is then found by adding all the carbonate species and the hydroxide, and then
subtracting the hydrogen ions. When using the units “mg/L as CaCO 3 ,” the terms are added
2
directly. The multiple of two for CO 3 has already been accounted for in the conversion.
2
Example 6-1. A water contains 100.0 mg/L CO 3 and 75.0 mg/L HCO 3 at a pH of 10. Calcu-
late the alkalinity exactly at 25 C. Approximate the alkalinity by ignoring [OH ] and [H ].
2
Solution. First, convert CO 3 , HCO 3 , OH , and H to mg/L as CaCO 3 .
The equivalent weights are
2
2
CO 3 : MW 60 n , EW 30
,
HCO 3 : MW 61 n , EW 61
1
,
1
1
H : MW ,n ,EW 1
OH : MW 17 n ,EW 17
,
1
10
From the pH of 10, [H ] 10 moles/L and the mg/L of the species is
3
)
mg/L of species ( moles/L GMW)(10 mg/g)
(
10 3 7
(10 moles/L )(1 g/mole )(10 mg/g ) 10 mgg/L of H
The OH concentration is determined from the ionization of water, that is
K w [OH ][H ]