Page 209 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 209
P2: KVU/KXT
QC: —/—
T1: IML
P1: KVU/KXT
June 22, 2007
AT029-04
AT029-Manual-v7.cls
AT029-Manual
4. CHARACTERIZATION OF RESERVOIR FLUIDS AND CRUDE OILS 189
From Eqs. (4.114), (4.117), and (4.118) one can derive the
following relation for calculation of parameter φ: 21:30 0.005 Crude Feed
Liquid Product
0.004
∞
p − p Vapor Product
s
F
(4.119) T F dT = 0
T
(1 − φ)p + φp s T
T ◦ PDF, F(T b ), 1/K 0.003
where the integration should be carried numerically and φ
may be determined by trial-and-error procedure. As will be 0.002
shown in Chapter 7, combination of Trouton’s rule for the heat
of vaporization and the Clasius–Clapeyron equation leads to
0.001
the following relation for the vapor pressure:
T
(4.120) p T s = p a exp 10.58 1 − 0
T s -200 0 200 400 600 800 1000
°
where T is the boiling point of each cut in the distribution Boiling Point, T b , C
s
model, T is the saturation temperature, and p a is the atmo-
FIG. 4.26—Predicted probability density functions
s
spheric pressure. Both T and T must be in K. By combining of feed, liquid, and vapor at 300 C for flash vaporiza-
◦
Eqs. (4.114) and (4.117) we get
tion of a Russian crude oil. Actual data are taken from
p Ref. [31].
L
(4.121) F = F F
T (1 − φ)p + φp s T T
p s
V
(4.122) F = T F F
T (1 − φ)p + φp s T where Eqs. (4.123)–(4.125) are equivalent to Eqs. (4.119),
T
(4.121), and (4.122) for ideal systems, respectively. Calcula-
After finding φ from Eq. (4.119), it can be substituted in the tion of equilibrium ratios from equations of state will be dis-
above equations to find density functions for the vapor and cussed in Chapters 6 and 9. Probability density functions in
liquid products. these equations may be expressed in terms of other character-
For evaluation and application of these equations, data on ization parameters such as molecular weight or carbon num-
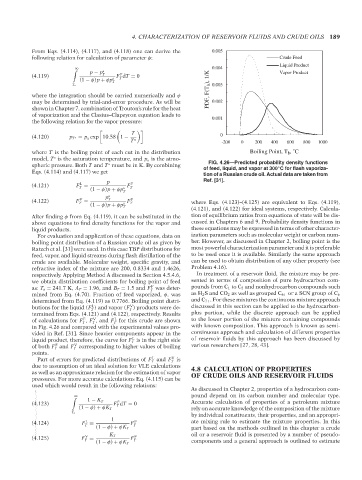
boiling point distribution of a Russian crude oil as given by ber. However, as discussed in Chapter 2, boiling point is the
Ratzch et al. [31] were used. In this case TBP distributions for most powerful characterization parameter and it is preferable
feed, vapor, and liquid streams during flash distillation of the to be used once it is available. Similarly the same approach
crude are available. Molecular weight, specific gravity, and can be used to obtain distribution of any other property (see
refractive index of the mixture are 200, 0.8334 and 1.4626, Problem 4.16).
respectively. Applying Method A discussed in Section 4.5.4.6, In treatment of a reservoir fluid, the mixture may be pre-
we obtain distribution coefficients for boiling point of feed sented in terms of composition of pure hydrocarbon com-
F
as: T o = 241.7K, A T = 1.96, and B T = 1.5 and F was deter- pounds from C 1 to C 5 and nonhydrocarbon compounds such
T
mined from Eq. (4.70). Fraction of feed vaporized, φ, was as H 2 S and CO 2 as well as grouped C 6+ or a SCN group of C 6
determined from Eq. (4.119) as 0.7766. Boiling point distri- and C 7+ . For these mixtures the continuous mixture approach
L
V
butions for the liquid (F ) and vapor (F ) products were de- discussed in this section can be applied to the hydrocarbon-
T T
termined from Eqs. (4.121) and (4.122), respectively. Results plus portion, while the discrete approach can be applied
V
L
F
of calculations for F , F , and F for this crude are shown to the lower portion of the mixture containing compounds
T T T
in Fig. 4.26 and compared with the experimental values pro- with known composition. This approach is known as semi-
vided in Ref. [31]. Since heavier components appear in the continuous approach and calculation of different properties
L
liquid product, therefore, the curve for F is in the right side of reservoir fluids by this approach has been discussed by
T
of both F and F corresponding to higher values of boiling various researchers [27, 28, 43].
F
V
T
T
points.
V
L
Part of errors for predicted distributions of F and F is
T
T
due to assumption of an ideal solution for VLE calculations 4.8 CALCULATION OF PROPERTIES
as well as an approximate relation for the estimation of vapor OF CRUDE OILS AND RESERVOIR FLUIDS
pressures. For more accurate calculations Eq. (4.115) can be
used which would result in the following relations:
As discussed in Chapter 2, properties of a hydrocarbon com-
pound depend on its carbon number and molecular type.
∞
1 − K T F Accurate calculation of properties of a petroleum mixture
(4.123) F dT = 0
T
(1 − φ) + φK T rely on accurate knowledge of the composition of the mixture
T ◦ by individual constituents, their properties, and an appropri-
1
L
(4.124) F = F F ate mixing rule to estimate the mixture properties. In this
T T
(1 − φ) + φK T part based on the methods outlined in this chapter a crude
V
(4.125) F = K T F T F oil or a reservoir fluid is presented by a number of pseudo-
T
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
(1 − φ) + φK T components and a general approach is outlined to estimate
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT