Page 24 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 24
T1: IML
QC: IML/FFX
P1: IML/FFX
P2: IML/FFX
AT029-Manual-v7.cls
June 22, 2007
AT029-Manual
14:26
AT029-01
4 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
Unsaturated compounds are more reactive than saturated hy-
drocarbons (without double bond). Olefins are uncommon in
crude oils due to their reactivity with hydrogen that makes
them saturated; however, they can be produced in refiner-
ies through cracking reactions. Olefins are valuable prod-
ucts of refineries and are used as the feed for petrochemical --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
Number of Isomers compounds with triple bonds such as acetylene (CH CH) are
plants to produce polymers such as polyethylene. Similarly
not found in crude oils because of their tendency to become
saturated [2].
Naphthenes or cycloalkanes are ring or cyclic saturated hy-
drocarbons with the general formula of C n H 2n . Cyclopentane
(C 5 H 10 ), cyclohexane (C 6 H 12 ), and their derivatives such as
n-alkylcyclopentanes are normally found in crude oils. Three
types of naphthenic compounds are shown below:
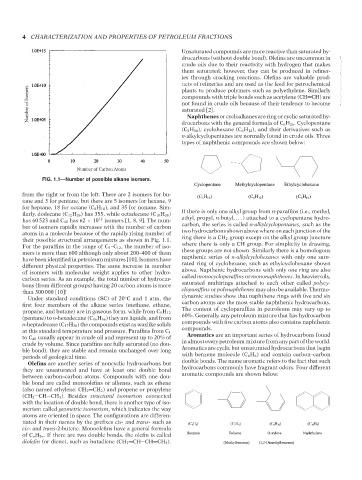
Number of Carbon Atoms
FIG. 1.1—Number of possible alkane isomers.
Cyclopentane Methylcyclopentane Ethylcyclohexane
from the right or from the left. There are 2 isomers for bu- (C 5 H 10 ) (C 6 H 12 ) (C 8 H 16 )
tane and 3 for pentane, but there are 5 isomers for hexane, 9
for heptane, 18 for octane (C 8 H 18 ), and 35 for nonane. Sim-
ilarly, dodecane (C 12 H 26 ) has 355, while octadecane (C 18 H 38 ) If there is only one alkyl group from n-paraffins (i.e., methyl,
has 60 523 and C 40 has 62 × 10 12 isomers [1, 8, 9]. The num- ethyl, propyl, n-butyl, ...) attached to a cyclopentane hydro-
ber of isomers rapidly increases with the number of carbon carbon, the series is called n-alkylcyclopentanes, such as the
atoms in a molecule because of the rapidly rising number of two hydrocarbons shown above where on each junction of the
their possible structural arrangements as shown in Fig. 1.1. ring there is a CH 2 group except on the alkyl group juncture
For the paraffins in the range of C 5 –C 12 , the number of iso- where there is only a CH group. For simplicity in drawing,
mers is more than 600 although only about 200–400 of them these groups are not shown. Similarly there is a homologous
have been identified in petroleum mixtures [10]. Isomers have napthenic series of n-alkylcyclohexanes with only one satu-
different physical properties. The same increase in number rated ring of cyclohexane, such as ethylcyclohexane shown
of isomers with molecular weight applies to other hydro- above. Napthenic hydrocarbons with only one ring are also
carbon series. As an example, the total number of hydrocar- called monocycloparaffins or mononaphthenes. In heavier oils,
bons (from different groups) having 20 carbon atoms is more saturated multirings attached to each other called polycy-
than 300 000 [10]! cloparaffins or polynaphthenes may also be available. Thermo-
Under standard conditions (SC) of 20 C and 1 atm, the dynamic studies show that naphthene rings with five and six
◦
first four members of the alkane series (methane, ethane, carbon atoms are the most stable naphthenic hydrocarbons.
The content of cycloparaffins in petroleum may vary up to
propane, and butane) are in gaseous form, while from C 5 H 12 60%. Generally, any petroleum mixture that has hydrocarbon
(pentane) to n-hexadecane (C 16 H 36 ) they are liquids, and from
n-heptadecane (C 17 H 38 ) the compounds exist as waxlike solids compounds with five carbon atoms also contains naphthenic
compounds.
at this standard temperature and pressure. Paraffins from C 1 Aromatics are an important series of hydrocarbons found
to C 40 usually appear in crude oil and represent up to 20% of
crude by volume. Since paraffins are fully saturated (no dou- in almost every petroleum mixture from any part of the world.
ble bond), they are stable and remain unchanged over long Aromatics are cyclic but unsaturated hydrocarbons that begin
periods of geological time. with benzene molecule (C 6 H 6 ) and contain carbon–carbon
Olefins are another series of noncyclic hydrocarbons but double bonds. The name aromatic refers to the fact that such
they are unsaturated and have at least one double bond hydrocarbons commonly have fragrant odors. Four different
between carbon–carbon atoms. Compounds with one dou- aromatic compounds are shown below:
ble bond are called monoolefins or alkenes, such as ethene
(also named ethylene: CH 2 CH 2 ) and propene or propylene
(CH 2 CH CH 3 ). Besides structural isomerism connected
with the location of double bond, there is another type of iso-
merism called geometric isomerism, which indicates the way
atoms are oriented in space. The configurations are differen-
tiated in their names by the prefixes cis- and trans- such as (C 6 H 6 ) (C 7 H 8 ) (C 8 H 10 ) (C 10 H 8 )
cis- and trans-2-butene. Monoolefins have a general formula
of C n H 2n . If there are two double bonds, the olefin is called Benzene Toluene O-xylene Naphthalene
diolefin (or diene), such as butadiene (CH 2 CH CH CH 2 ). (Methylbenzene) (1,2-Dimethylbenzene)
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT