Page 28 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 28
T1: IML
P2: IML/FFX
QC: IML/FFX
P1: IML/FFX
14:26
AT029-Manual
AT029-01
AT029-Manual-v7.cls
June 22, 2007
8 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
TABLE 1.3—Some petroleum fractions produced from distillation columns.
Approximate boiling range
Petroleum fraction Approximate hydrocarbon range ◦ C ◦ F
Light gases C 2 –C 4 −90 to 1 −130–30
Gasoline (light and heavy) C 4 –C 10 −1–200 30–390
Naphthas (light and heavy) C 4 –C 11 −1–205 30–400
Jet fuel C 9 –C 14 150–255 300–490
Kerosene C 11 –C 14 205–255 400–490
Diesel fuel C 11 –C 16 205–290 400–550
Light gas oil C 14 –C 18 255–315 490–600
Heavy gas oil C 18 –C 28 315–425 600–800
Wax C 18 –C 36 315–500 600–930
Lubricating oil >C 25 >400 >750
Vacuum gas oil C 28 –C 55 425–600 800–1100
Residuum >C 55 >600 >1100
Information given in this table is obtained from different sources [1, 18, 19].
range of −180 to −80 C(−260 to −40 F), which corresponds molecular weight multiring aromatics. The vacuum residuum
◦
◦
to the boiling point of methane and ethane. This mixture, may be mixed with lighter products to produce a more valu-
which is in the form of gas and is known as fuel gas, is actu- able blend.
ally a petroleum fraction. In fact, during distillation a crude Distillation of a crude oil can also be performed in the lab-
is converted into a series of petroleum fractions where each oratory to divide the mixture into many narrow boiling point
one is a mixture of a limited number of hydrocarbons with a range fractions with a boiling range of about 10 C. Such nar-
◦
specific range of boiling point. Fractions with a wider range row range fractions are sometimes referred to as petroleum
of boiling points contain greater numbers of hydrocarbons. cuts. When boiling points of all the cuts in a crude are known, --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
All fractions from a distillation column have a known boiling then the boiling point distribution (distillation curve) of the
range, except the residuum for which the upper boiling point
is usually not known. The boiling point of the heaviest com-
ponent in a crude oil is not really known, but it is quite high. 60
The problem of the nature and properties of the heaviest com-
pounds in crude oils and petroleum residuum is still under
investigation by researchers [16, 17]. Theoretically, it can be
assumed that the boiling point of the heaviest component in a Vacuum
crude oil is infinity. Atmospheric residue has compounds with 50 Residuum
carbon number greater than 25, while vacuum residue has - 655
compounds with carbon number greater than 50 (M > 800).
Some of the petroleum fractions produced from distillation
columns with their boiling point ranges and applications are 40 Vacuum
Gas Oil
given in Table 1.3. The boiling point and equivalent carbon
number ranges given in this table are approximate and they
may vary according to the desired specific product. For ex-
ample, the light gases fraction is mainly a mixture of ethane, -
propane, and butane; however, some heavier compounds Carbon Number 30 Boiling Point, °C
(C 5+ ) may exist in this fraction. The fraction is further frac- Heavy
tionated to obtain ethane (a fuel gas) and propane and butane Atmospheric Distillation 46.1% Gas Oil
(petroleum gases). The petroleum gases are liquefied to get
liquefied petroleum gas (LPG) used for home cooking pur- - 345
Light
poses. In addition the isobutane may be separated for the gas 20 Gas Oil
mixture to be used for improving vapor pressure characteris-
- 205
tics (volatility) of gasoline in cold weathers. These fractions
Kerosene
may go through further processes to produce desired prod-
ucts. For example, gas oil may go through a cracking process 10
to obtain more gasoline. Since distillation is not a perfect sep- Naphtha
aration process, the initial and final boiling points for each
Vacuum Distillation 53.9%
fraction are not exact and especially the end points are ap-
proximate values. Fractions may be classified as narrow or -- 90
wide depending on their boiling point range. As an example, 0
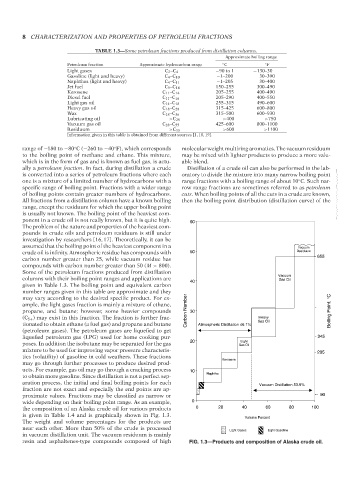
the composition of an Alaska crude oil for various products 0 20 40 60 80 100
is given in Table 1.4 and is graphically shown in Fig. 1.3. Volume Percent
The weight and volume percentages for the products are
near each other. More than 50% of the crude is processed Light Gases Light Gasoline
in vacuum distillation unit. The vacuum residuum is mainly
resin and asphaltenes-type compounds composed of high FIG. 1.3—Products and composition of Alaska crude oil.
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT