Page 25 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 25
T1: IML
P2: IML/FFX
P1: IML/FFX
QC: IML/FFX
AT029-Manual-v7.cls
AT029-Manual
14:26
AT029-01
June 22, 2007
In the above structures, on each junction on the benzene
ring where there are three bonds, there is only a group of CH, 1. INTRODUCTION 5
while at the junction with an alkylgroup (i.e., toluene) there
is only a C atom. Although benzene has three carbon–carbon
double bonds, it has a unique arrangement of electrons that
allows benzene to be relatively unreactive. Benzene is, how-
ever, known to be a cancer-inducing compound [2]. For this
reason, the amount of benzene allowed in petroleum prod-
ucts such as gasoline or fuel oil is limited by government
regulations in many countries. Under SC, benzene, toluene,
and xylene are in liquid form while naphthalene is in a solid
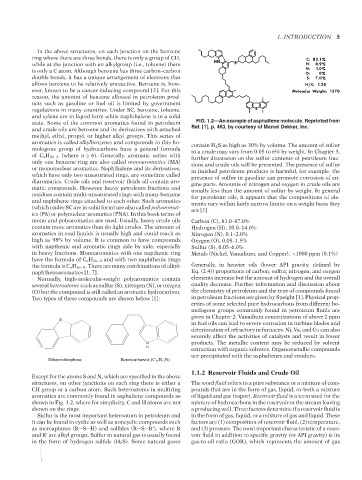
state. Some of the common aromatics found in petroleum FIG. 1.2—An example of asphaltene molecule. Reprinted from
and crude oils are benzene and its derivatives with attached Ref. [1], p. 463, by courtesy of Marcel Dekker, Inc.
methyl, ethyl, propyl, or higher alkyl groups. This series of
aromatics is called alkylbenzenes and compounds in this ho- contain H 2 S as high as 30% by volume. The amount of sulfur
mologous group of hydrocarbons have a general formula in a crude may vary from 0.05 to 6% by weight. In Chapter 3,
of C n H 2n−6 (where n ≥ 6). Generally, aromatic series with further discussion on the sulfur contents of petroleum frac-
only one benzene ring are also called monoaromatics (MA) tions and crude oils will be presented. The presence of sulfur
or mononuclear aromatics. Naphthalene and its derivatives, in finished petroleum products is harmful, for example, the
which have only two unsaturated rings, are sometime called presence of sulfur in gasoline can promote corrosion of en-
diaromatics. Crude oils and reservoir fluids all contain aro- gine parts. Amounts of nitrogen and oxygen in crude oils are
matic compounds. However, heavy petroleum fractions and usually less than the amount of sulfur by weight. In general
residues contain multi-unsaturated rings with many benzene for petroleum oils, it appears that the compositions of ele-
and naphthene rings attached to each other. Such aromatics ments vary within fairly narrow limits; on a weight basis they
(which under SC are in solid form) are also called polyaromat- are [1]
ics (PA) or polynuclear aromatics (PNA). In this book terms of
mono and polyaromatics are used. Usually, heavy crude oils Carbon (C), 83.0–87.0%
contain more aromatics than do light crudes. The amount of Hydrogen (H), 10.0–14.0%
aromatics in coal liquids is usually high and could reach as Nitrogen (N), 0.1–2.0%
high as 98% by volume. It is common to have compounds Oxygen (O), 0.05–1.5%
with napthenic and aromatic rings side by side, especially Sulfur (S), 0.05–6.0%
in heavy fractions. Monoaromatics with one napthenic ring Metals (Nickel, Vanadium, and Copper), <1000 ppm (0.1%)
have the formula of C n H 2n−8 and with two naphthenic rings
the formula is C n H 2n−8 . There are many combinations of alkyl- Generally, in heavier oils (lower API gravity, defined by
naphthenoaromatics [1, 7]. Eq. (2.4)) proportions of carbon, sulfur, nitrogen, and oxygen
Normally, high-molecular-weight polyaromatics contain elements increase but the amount of hydrogen and the overall
several heteroatoms such as sulfur (S), nitrogen (N), or oxygen quality decrease. Further information and discussion about
(O) but the compound is still called an aromatic hydrocarbon. the chemistry of petroleum and the type of compounds found
Two types of these compounds are shown below [1]: in petroleum fractions are given by Speight [1]. Physical prop-
erties of some selected pure hydrocarbons from different ho-
mologous groups commonly found in petroleum fluids are
given in Chapter 2. Vanadium concentrations of above 2 ppm
S
in fuel oils can lead to severe corrosion in turbine blades and
deterioration of refractory in furnaces. Ni, Va, and Cu can also
severely affect the activities of catalysts and result in lower
N products. The metallic content may be reduced by solvent
H
extraction with organic solvents. Organometallic compounds
are precipitated with the asphaltenes and residues.
Dibenzothiophene Benzocarbazole (C 16 H 11 N)
1.1.2 Reservoir Fluids and Crude Oil
Except for the atoms S and N, which are specified in the above
structures, on other junctions on each ring there is either a The word fluid refers to a pure substance or a mixture of com-
CH group or a carbon atom. Such heteroatoms in multiring pounds that are in the form of gas, liquid, or both a mixture
aromatics are commonly found in asphaltene compounds as of liquid and gas (vapor). Reservoir fluid is a term used for the
shown in Fig. 1.2, where for simplicity, C and H atoms are not mixture of hydrocarbons in the reservoir or the stream leaving
shown on the rings. a producing well. Three factors determine if a reservoir fluid is
Sulfur is the most important heteroatom in petroleum and in the form of gas, liquid, or a mixture of gas and liquid. These
it can be found in cyclic as well as noncyclic compounds such factors are (1) composition of reservoir fluid, (2) temperature,
as mercaptanes (R S H) and sulfides (R S R ), where R and (3) pressure. The most important characteristic of a reser-
and R are alkyl groups. Sulfur in natural gas is usually found voir fluid in addition to specific gravity (or API gravity) is its
in the form of hydrogen sulfide (H 2 S). Some natural gases gas-to-oil ratio (GOR), which represents the amount of gas
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT