Page 360 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 360
P1: JDW
AT029-Manual
14:25
June 22, 2007
AT029-Manual-v7.cls
AT029-08
340 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
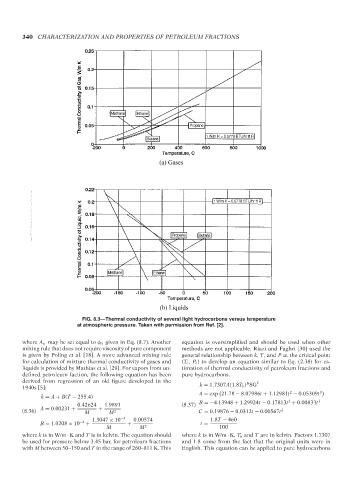
FIG. 8.3—Thermal conductivity of several light hydrocarbons versus temperature
at atmospheric pressure. Taken with permission from Ref. [2].
where A ij may be set equal to φ ij given in Eq. (8.7). Another equation is oversimplified and should be used when other
mixing rule that does not require viscosity of pure component methods are not applicable. Riazi and Faghri [30] used the
is given by Poling et al. [18]. A more advanced mixing rule general relationship between k, T, and P at the critical point
for calculation of mixture thermal conductivity of gases and (T c , P c ) to develop an equation similar to Eq. (2.38) for es-
liquids is provided by Mathias et al. [29]. For vapors from un- timation of thermal conductivity of petroleum fractions and
defined petroleum faction, the following equation has been pure hydrocarbons.
derived from regression of an old figure developed in the B C
1940s [5]: k = 1.7307A(1.8T b ) SG
2
A = exp 21.78 − 8.07986t + 1.12981t − 0.05309t 3
k = A + B(T − 255.4) 2 3
0.42624 1.9891 (8.37) B =−4.13948 + 1.29924t − 0.17813t + 0.00833t
(8.36) A = 0.00231 + M + M 2 C = 0.19876 − 0.0312t − 0.00567t 2
1.3047 × 10 −4 0.00574 1.8T − 460
B = 1.0208 × 10 −4 + + t =
M M 2 100
where k is in W/m · K and T is in kelvin. The equation should where k is in W/m · K, T b and T are in kelvin. Factors 1.7307
be used for pressure below 3.45 bar, for petroleum fractions and 1.8 come from the fact that the original units were in
with M between 50–150 and T in the range of 260–811 K. This English. This equation can be applied to pure hydrocarbons
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT