Page 363 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 363
P1: JDW
AT029-Manual
June 22, 2007
AT029-Manual-v7.cls
14:25
AT029-08
8. APPLICATIONS: ESTIMATION OF TRANSPORT PROPERTIES 343
TABLE 8.6—Liquid thermal conductivity of some pure compounds at their normal
melting and boiling points [5].
No. Compound T M ,K k at T M , W/mK T b ,K k at T b , W/mK
1 Methane 90.69 0.2247 111.66 0.1883
2 Propane 85.47 0.2131 231.11 0.1289
3 n-Butane 134.86 0.1869 272.65 0.1176
4 n-Pentane 143.42 0.1783 309.22 0.1086
5 n-Hexane 177.83 0.1623 341.88 0.1042
6 2-Methylpentane 119.55 0.1600 333.41 0.1000
7 3-Methylpentane 110.25 0.1646 336.42 0.1010
8 n-Heptane 182.57 0.1599 371.58 0.1025
9 n-Octane 216.38 0.1520 398.82 0.0981
10 2.24-Trimethylpentane 165.78 0.1284 372.39 0.0815
11 n-Nonane 219.66 0.1512 423.97 0.0972
12 n-Decane 243.51 0.1456 447.31 0.0946
13 n-Undecane 247.57 0.1461 469.04 0.0930
14 n-Dodecane 263.57 0.1436 489.47 0.0909
15 n-Tridecane 267.76 0.1441 508.62 0.0896
16 n-Tetradecane 279.01 0.1423 526.73 0.0882
17 n-Pentadecane 283.07 0.1446 543.83 0.0874
18 n-Hexadecane 291.31 0.1438 560.02 0.0849
19 n-Heptadecane 295.13 0.1441 575.26 0.0819
20 n-Octadecane 301.31 0.1460 589.86 0.0810
21 n-Nonadecane 305.04 0.1453 603.05 0.0797
22 n-Eicosane 309.58 0.1488 616.94 0.0801
23 n-Heneicosane 313.35 0.1499 629.66 0.0799
24 n-Docosane 317.15 0.1513 641.75 0.0809
25 n-Tricosane 320.65 0.1516 653.35 0.0811
26 n-Tetracosane 323.75 0.1530 664.45 0.0819
27 Cyclopentane 179.31 0.1584 322.40 0.1198
28 Methylcyclopentane 130.73 0.1605 344.96 0.1071
29 Cyclohexane 279.69 0.1282 353.87 0.1096
30 Methylcyclohexane 146.58 0.1449 374.04 0.0935
31 Cyclohexane 169.67 0.1653 356.12 0.1167
32 Benzene 278.68 0.1494 353.24 0.1266
33 Methylbenzene (toluene) 178.18 0.1616 383.78 0.1117
34 Ethylbenzene 178.20 0.1576 409.35 0.1025
35 n-Propylbenzene 173.55 0.1528 432.39 0.1014
36 n-Butylbenzene 185.30 0.1501 456.46 0.0957
L
where V 25 is the liquid molar volume at 25 C (298 K) in API-TDB [5]. This equation can be used for temperatures at
◦
3
L
cm /mol. For some compounds these values of V L are given T r < 0.8 and pressure below 35 bar. For estimation of k at
25
in Table 6.10. M is the molecular weight in g/mol and k L temperatures above normal boiling point (compressed or sat-
is desired liquid thermal conductivity at T in W/m · K. Av- urated liquids), there are a number of methods that use re-
erage error for this equation is about 5% as reported in the duced density ρ r as a correlating parameter [5, 8]. Riazi and
Faghri [30] also developed a method similar to Eq. (8.37) for
prediction of thermal conductivity of liquid hydrocarbons for
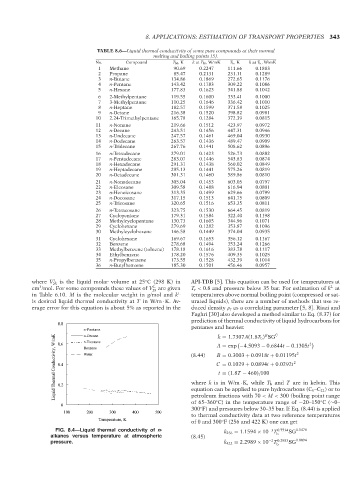
0.8
n-Pentane pentanes and heavier. B C 2
Liquid Thermal Conductivity, W/mK 0.4 Water (8.44) C = 0.1029 + 0.0894t + 0.0292t 2 2
n-Decane
k = 1.7307A(1.8T b ) SG
n-Eicosane
0.6
A = exp −4.5093 − 0.6844t − 0.1305t
Benzene
B = 0.3003 + 0.0918t + 0.01195t
t = (1.8T − 460)/100
where k is in W/m · K, while T b and T are in kelvin. This
0.2
equation can be applied to pure hydrocarbons (C 5 –C 22 )orto
petroleum fractions with 70 < M < 300 (boiling point range
of 65–360 C) in the temperature range of −20–150 C(∼0–
◦
◦
0
300 F) and pressures below 30–35 bar. If Eq. (8.44) is applied
◦
100 200 300 400 500
to thermal conductivity data at two reference temperatures
Temperature, K
of 0 and 300 F (256 and 422 K) one can get
◦
FIG. 8.4—Liquid thermal conductivity of n- k 256 = 1.1594 × 10 T 0.7534 SG 0.5478
−3
alkanes versus temperature at atmospheric (8.45) b
−2
pressure. k 422 = 2.2989 × 10 T 0.2983 SG 0.0094
b
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT