Page 175 - Advanced Gas Turbine Cycles
P. 175
Chapter 8. Novel gas turbine cycles 141
COYBUSTlON
7.52 N,
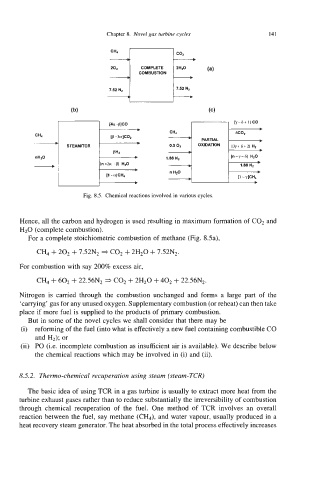
Fig. 8.5. Chemical reactions involved in various cycles.
Hence, all the carbon and hydrogen is used resulting in maximum formation of C02 and
H20 (complete combustion).
For a complete stoichiometric combustion of methane (Fig. Ma),
Cb + 202 + 7.52N2 * C02 + 2H20 + 7.52N2.
For combustion with say 200% excess air,
CH4 + 602 + 22.56N2 * C02 + 2H20 + 402 + 22.56N2.
Nitrogen is carried through the combustion unchanged and forms a large part of the
‘carrying’ gas for any unused oxygen. Supplementary combustion (or reheat) can then take
place if more fuel is supplied to the products of primary combustion.
But in some of the novel cycles we shall consider that there may be
(i) reforming of the fuel (into what is effectively a new fuel containing combustible CO
and H2); or
(ii) PO (i.e. incomplete combustion as insufficient air is available). We describe below
the chemical reactions which may be involved in (i) and (ii).
8.5.2. Thermo-chemical recuperation using steam (steam-TCR)
The basic idea of using TCR in a gas turbine is usually to extract more heat from the
turbine exhaust gases rather than to reduce substantially the irreversibility of combustion
through chemical recuperation of the fuel. One method of TCR involves an overall
reaction between the fuel, say methane (Ch), and water vapour, usually produced in a
heat recovery steam generator. The heat absorbed in the total process effectively increases