Page 1006 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1006
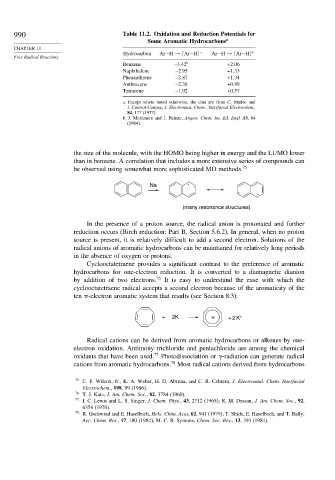
990 Table 11.2. Oxidation and Reduction Potentials for
Some Aromatic Hydrocarbons a
CHAPTER 11
Hydrocarbon Ar−H → [Ar−H] − Ar−H → [Ar−H] +
Free Radical Reactions
Benzene –3.42 b +2.06
Naphthalene –2.95 +1.33
Phenanthrene –2.87 +1.34
Anthracene –2.36 +0.89
Tetracene –1.92 +0.57
a. Except where noted otherwise, the data are from C. Madec and
J. Courtot-Coupez, J. Electroanal. Chem., Interfacial Electrochem.,
84, 177 (1977).
b. J. Mortensen and J. Heinze, Angew. Chem. Int. Ed. Engl .13,84
(1984).
the size of the molecule, with the HOMO being higher in energy and the LUMO lower
than in benzene. A correlation that includes a more extensive series of compounds can
be observed using somewhat more sophisticated MO methods. 75
.
Na -
.
-
(many resonance structures)
In the presence of a proton source, the radical anion is protonated and further
reduction occurs (Birch reduction; Part B, Section 5.6.2). In general, when no proton
source is present, it is relatively difficult to add a second electron. Solutions of the
radical anions of aromatic hydrocarbons can be maintained for relatively long periods
in the absence of oxygen or protons.
Cyclooctatetraene provides a significant contrast to the preference of aromatic
hydrocarbons for one-electron reduction. It is converted to a diamagnetic dianion
by addition of two electrons. 76 It is easy to understand the ease with which the
cyclooctatetraene radical accepts a second electron because of the aromaticity of the
ten -electron aromatic system that results (see Section 8.3).
+ 2K = + 2 K +
Radical cations can be derived from aromatic hydrocarbons or alkenes by one-
electron oxidation. Antimony trichloride and pentachloride are among the chemical
oxidants that have been used. 77 Photodissociation or -radiation can generate radical
78
cations from aromatic hydrocarbons. Most radical cations derived from hydrocarbons
75 C. F. Wilcox, Jr., K. A. Weber, H. D. Abruna, and C. R. Cabrera, J. Electroanal. Chem. Interfacial
Electrochem., 198, 99 (1986).
76
T. J. Katz, J. Am. Chem. Soc., 82, 3784 (1960).
77 I. C. Lewis and L. S. Singer, J. Chem. Phys., 43, 2712 (1965); R. M. Dessau, J. Am. Chem. Soc., 92,
6356 (1970).
78
R. Gschwind and E. Haselbach, Helv. Chim. Acta, 62, 941 (1979); T. Shida, E. Haselbach, and T. Bally,
Acc. Chem. Res., 17, 180 (1984); M. C. R. Symons, Chem. Soc. Rev., 13, 393 (1984).