Page 1002 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1002
986 H .
Y Z
CHAPTER 11 H
Free Radical Reactions
Y Z = –CH CH , CH O, C N
2
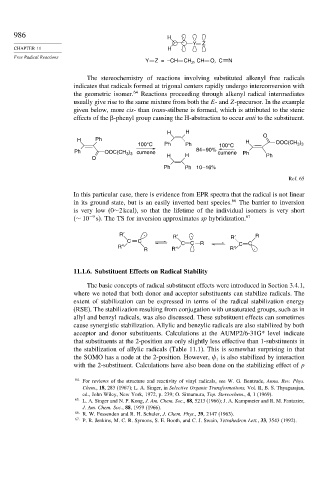
The stereochemistry of reactions involving substituted alkenyl free radicals
indicates that radicals formed at trigonal centers rapidly undergo interconversion with
the geometric isomer. 64 Reactions proceeding through alkenyl radical intermediates
usually give rise to the same mixture from both the E- and Z-precursor. In the example
given below, more cis- than trans-stilbene is formed, which is attributed to the steric
effects of the -phenyl group causing the H-abstraction to occur anti to the substituent.
H H
O
H Ph H )
100°C Ph Ph 100°C OOC(CH 3 3
Ph OOC(CH ) H 84 – 90% cumene Ph
3 3 cumene
O H Ph
Ph Ph 10 –16%
Ref. 65
In this particular case, there is evidence from EPR spectra that the radical is not linear
in its ground state, but is an easily inverted bent species. 66 The barrier to inversion
is very low (0∼2kcal), so that the lifetime of the individual isomers is very short
(∼ 10 −9 s). The TS for inversion approximates sp hybridization. 67
R′ . R′ . R′ R
C C C C
R″ R R″ C C
R R″ .
11.1.6. Substituent Effects on Radical Stability
The basic concepts of radical substituent effects were introduced in Section 3.4.1,
where we noted that both donor and acceptor substituents can stabilize radicals. The
extent of stabilization can be expressed in terms of the radical stabilization energy
(RSE). The stabilization resulting from conjugation with unsaturated groups, such as in
allyl and benzyl radicals, was also discussed. These substituent effects can sometimes
cause synergistic stabilization. Allylic and benzylic radicals are also stabilized by both
acceptor and donor substituents. Calculations at the AUMP2/6-31G* level indicate
that substituents at the 2-position are only slightly less effective than 1-substituents in
the stabilization of allylic radicals (Table 11.1). This is somewhat surprising in that
the SOMO has a node at the 2-position. However, is also stabilized by interaction
1
with the 2-substituent. Calculations have also been done on the stabilizing effect of p
64
For reviews of the structure and reactivity of vinyl radicals, see W. G. Bentrude, Annu. Rev. Phys.
Chem., 18, 283 (1967); L. A. Singer, in Selective Organic Transformations, Vol. II, B. S. Thyagarajan,
ed., John Wiley, New York, 1972, p. 239; O. Simamura, Top. Stereochem., 4, 1 (1969).
65 L. A. Singer and N. P. Kong, J. Am. Chem. Soc., 88, 5213 (1966); J. A. Kampmeier and R. M. Fantazier,
J. Am. Chem. Soc., 88, 1959 (1966).
66 R. W. Fessenden and R. H. Schuler, J. Chem. Phys., 39, 2147 (1963).
67
P. R. Jenkins, M. C. R. Symons, S. E. Booth, and C. J. Swain, Tetrahedron Lett., 33, 3543 (1992).