Page 1007 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1007
have limited stability, but ESR spectral parameters have permitted structural charac- 991
terization. 79 The radical cations can be generated electrochemically and the oxidation
potentials are included in Table 11.2. The potentials correlate with the HOMO levels of SECTION 11.1
the hydrocarbons. The higher the HOMO, the more easily the hydrocarbon is oxidized. Generation and
Characterization of Free
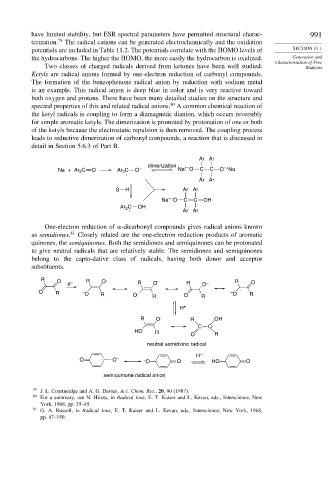
Two classes of charged radicals derived from ketones have been well studied. Radicals
Ketyls are radical anions formed by one-electron reduction of carbonyl compounds.
The formation of the benzophenone radical anion by reduction with sodium metal
is an example. This radical anion is deep blue in color and is very reactive toward
both oxygen and protons. There have been many detailed studies on the structure and
80
spectral properties of this and related radical anions. A common chemical reaction of
the ketyl radicals is coupling to form a diamagnetic dianion, which occurs reversibly
for simple aromatic ketyls. The dimerization is promoted by protonation of one or both
of the ketyls because the electrostatic repulsion is then removed. The coupling process
leads to reductive dimerization of carbonyl compounds, a reaction that is discussed in
detail in Section 5.6.3 of Part B.
Ar Ar
dimerization +– –+
Na + Ar 2 C O Ar C . O – Na O C C O Na
2
Ar Ar
S H Ar Ar
+–
Na O C C OH
C OH
Ar 2
. Ar Ar
One-electron reduction of -dicarbonyl compounds gives radical anions known
as semidiones. 81 Closely related are the one-electron reduction products of aromatic
quinones, the semiquinones. Both the semidiones and semiquinones can be protonated
to give neutral radicals that are relatively stable. The semidiones and semiquinones
belong to the capto-dative class of radicals, having both donor and acceptor
substituents.
R O e – R O . R O – R O – R O
O R – O R O . R O . R – O . R
H +
R O . R OH
C C·
HO R
O R
neutral semidione radical
H +
O . O – – O . O HO . O
semiquinone radical anion
79
J. L. Courtneidge and A. G. Davies, Acc. Chem. Res., 20, 90 (1987).
80 For a summary, see N. Hirota, in Radical Ions, E. T. Kaiser and L. Kevan, eds., Interscience, New
York, 1968, pp. 35–85.
81
G. A. Russell, in Radical Ions, E. T. Kaiser and L. Kevan, eds., Interscience, New York, 1968,
pp. 87–150.