Page 1164 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1164
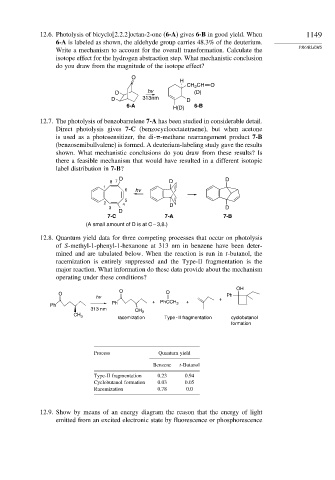
12.6. Photolysis of bicyclo[2.2.2]octan-2-one (6-A) gives 6-B in good yield. When 1149
6-A is labeled as shown, the aldehyde group carries 48.3% of the deuterium.
PROBLEMS
Write a mechanism to account for the overall transformation. Calculate the
isotope effect for the hydrogen abstraction step. What mechanistic conclusion
do you draw from the magnitude of the isotope effect?
O
H
CH CH O
2
D hv (D)
D 313nm D
6-A 6-B
H(D)
12.7. The photolysis of benzobarrelene 7-A has been studied in considerable detail.
Direct photolysis gives 7-C (benzocyclooctatetraene), but when acetone
is used as a photosensitizer, the di- -methane rearrangement product 7-B
(benzosemibullvalene) is formed. A deuterium-labeling study gave the results
shown. What mechanistic conclusions do you draw from these results? Is
there a feasible mechanism that would have resulted in a different isotopic
label distribution in 7-B?
D D
8 7 D
1
6 hv
5
2 4 D
3 D
D
7-C 7-A 7-B
(A small amount of D is at C – 3,8.)
12.8. Quantum yield data for three competing processes that occur on photolysis
of S-methyl-1-phenyl-1-hexanone at 313 nm in benzene have been deter-
mined and are tabulated below. When the reaction is run in t-butanol, the
racemization is entirely suppressed and the Type-II fragmentation is the
major reaction. What information do these data provide about the mechanism
operating under these conditions?
OH
O
O O Ph
hv +
Ph + PhCCH 3 +
Ph
313 nm
CH 3
CH 3
racemization Type - II fragmentation cyclobutanol
formation
Process Quantum yield
Benzene t-Butanol
Type-II fragmentation 0.23 0.94
Cyclobutanol formation 0.03 0.05
Racemization 0.78 0.0
12.9. Show by means of an energy diagram the reason that the energy of light
emitted from an excited electronic state by fluorescence or phosphorescence