Page 1165 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1165
1150 is of lower energy than the exciting radiation. Would you expect the shift in
energy to be more pronounced for fluorescence or phosphorescence? Explain?
CHAPTER 12 12.10. cis-2-Propyl-4-t-butylcyclohexanone undergoes cleavage to 4-t-butyl-
Photochemistry cyclohexanone on photolysis. The trans isomer does not undergo fragmentation
directly, but is converted to the cis isomer, which then fragments. The
trans → cis isomerization is quenched by 1,3-pentadiene, but the photofrag-
mentation is not. Offer an explanation of this pronounced stereochemical
effect.
12.11. The quantum yield for formation of 3-methylcyclobutene from E-1,3-
pentadiene by 254 nm radiation is ten times greater than for cyclization of
the Z-isomer. 1,3-Dimethylcyclopropene is also formed but the difference in
for this reactions is only a factor of two. Offer an explanation for these
differences in terms of energy surfaces that are involved.
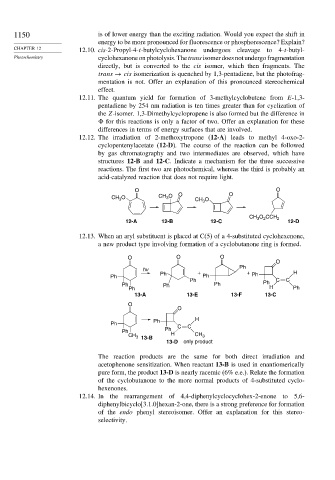
12.12. The irradiation of 2-methoxytropone (12-A) leads to methyl 4-oxo-2-
cyclopentenylacetate (12-D). The course of the reaction can be followed
by gas chromatography and two intermediates are observed, which have
structures 12-B and 12-C. Indicate a mechanism for the three successive
reactions. The first two are photochemical, whereas the third is probably an
acid-catalyzed reaction that does not require light.
O O
CH O CH O O CH 3 O O
3
3
CH O CCH 2
2
3
12-A 12-B 12-C 12-D
12.13. When an aryl substituent is placed at C(5) of a 4-substituted cyclohexenone,
a new product type involving formation of a cyclobutanone ring is formed.
O O O
O
Ph
hv
Ph + + Ph H
Ph Ph
Ph Ph C C
Ph Ph Ph H
Ph Ph
13-A 13-E 13-F 13-C
O
O
Ph H
Ph
Ph C C
Ph H
CH 3 13-B CH 3
13-D only product
The reaction products are the same for both direct irradiation and
acetophenone sensitization. When reactant 13-B is used in enantiomerically
pure form, the product 13-D is nearly racemic (6% e.e.). Relate the formation
of the cyclobutanone to the more normal products of 4-substituted cyclo-
hexenones.
12.14. In the rearrangement of 4,4-diphenylcyclocyclohex-2-enone to 5,6-
diphenylbicyclo[3.1.0]hexan-2-one, there is a strong preference for formation
of the endo phenyl stereoisomer. Offer an explanation for this stereo-
selectivity.