Page 1166 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1166
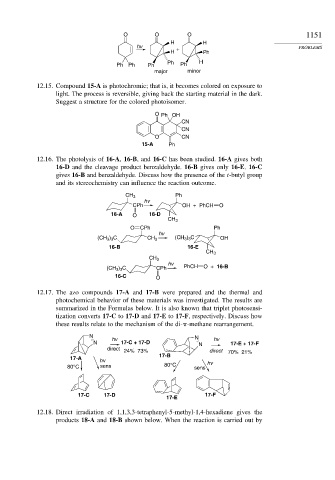
O O O 1151
H H
hv + PROBLEMS
H Ph
Ph Ph Ph Ph Ph H
major minor
12.15. Compound 15-A is photochromic; that is, it becomes colored on exposure to
light. The process is reversible, giving back the starting material in the dark.
Suggest a structure for the colored photoisomer.
O Ph OH
CN
CN
O CN
15-A Ph
12.16. The photolysis of 16-A, 16-B, and 16-C has been studied. 16-A gives both
16-D and the cleavage product benzaldehyde. 16-B gives only 16-E. 16-C
gives 16-B and benzaldehyde. Discuss how the presence of the t-butyl group
and its stereochemistry can influence the reaction outcome.
CH 3 Ph
hv
CPh OH + PhCH O
16-A O 16-D
CH 3
O CPh Ph
hv
(CH 3 ) 3 C CH 3 (CH 3 ) 3 C OH
16-B 16-E
CH 3
CH 3
hv
(CH 3 ) 3 C CPh PhCH O + 16-B
16-C O
12.17. The azo compounds 17-A and 17-B were prepared and the thermal and
photochemical behavior of these materials was investigated. The results are
summarized in the Formulas below. It is also known that triplet photosensi-
tization converts 17-C to 17-D and 17-E to 17-F, respectively. Discuss how
these results relate to the mechanism of the di- -methane rearrangement.
N N
hv hv
N 17-C + 17-D N 17-E + 17-F
direct 24% 73% direct 70% 21%
17-B
17-A
h v hv
80°C sens 80°C sens
17-C 17-D 17-F
17-E
12.18. Direct irradiation of 1,1,3,3-tetraphenyl-5-methyl-1,4-hexadiene gives the
products 18-A and 18-B shown below. When the reaction is carried out by