Page 1167 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1167
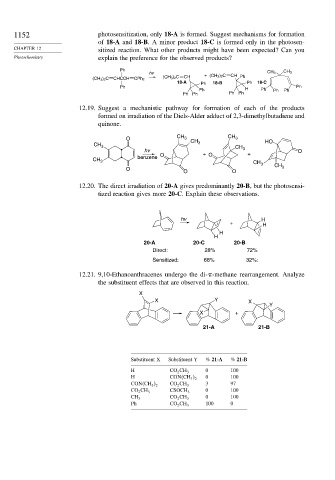
1152 photosensitization, only 18-A is formed. Suggest mechanisms for formation
of 18-A and 18-B. A minor product 18-C is formed only in the photosen-
CHAPTER 12 sitized reaction. What other products might have been expected? Can you
Photochemistry explain the preference for the observed products?
Ph
hv CH 3 CH 3
(CH 3 ) 2 C CHCCH CPh 2 (CH 3 ) 2 C CH + (CH 3) 2C CH Ph
18-A Ph 18-B Ph 18-C
Ph Ph
Ph H Ph Ph Ph
Ph Ph Ph Ph
12.19. Suggest a mechanistic pathway for formation of each of the products
formed on irradiation of the Diels-Alder adduct of 2,3-dimethylbutadiene and
quinone.
O CH 3 CH 3
CH 3 HO
CH 3
CH 3
hv O
O + O +
benzene
CH 3
CH 3
CH 3
O
O O
12.20. The direct irradiation of 20-A gives predominantly 20-B, but the photosensi-
tized reaction gives more 20-C. Explain these observations.
hv H
+ H
H
H
20-A 20-C 20-B
Direct: 28% 72%
Sensitized: 68% 32%:
12.21. 9,10-Ethanoanthracenes undergo the di- -methane rearrangement. Analyze
the substituent effects that are observed in this reaction.
X
X Y X
Y
X +
21-A 21-B
Substituent X Substituent Y % 21-A % 21-B
H CO 2 CH 3 0 100
H CON CH 3 2 0 100
3 97
CON CH 3 2 CO 2 CH 3
0 100
CO 2 CH 3 CSOCH 3
0 100
CH 3 CO 2 CH 3
Ph CO 2 CH 3 100 0