Page 1159 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1159
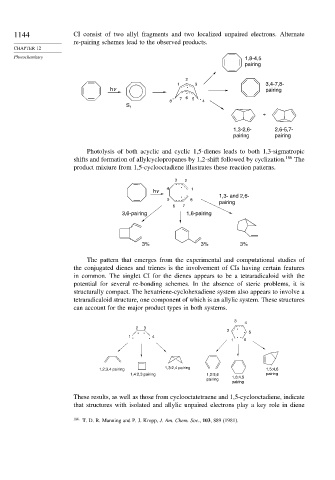
1144 CI consist of two allyl fragments and two localized unpaired electrons. Alternate
re-pairing schemes lead to the observed products.
CHAPTER 12
Photochemistry 1,8-4,5
pairing
2
1 . 3 3,4-7,8-
hv pairing
.
. 7 6 5 .
8 4
S 1
+
1,3-2,6- 2,6-5,7-
pairing pairing
Photolysis of both acyclic and cyclic 1,5-dienes leads to both 1,3-sigmatropic
shifts and formation of allylcyclopropanes by 1,2-shift followed by cyclization. 186 The
product mixture from 1,5-cyclooctadiene illustrates these reaction patterns.
3 2
.
4 1
hv
. 1,3- and 2,6-
5 8 pairing
6 7
3,6-pairing 1,6-pairing
3% 3% 3%
The pattern that emerges from the experimental and computational studies of
the conjugated dienes and trienes is the involvement of CIs having certain features
in common. The singlet CI for the dienes appears to be a tetraradicaloid with the
potential for several re-bonding schemes. In the absence of steric problems, it is
structurally compact. The hexatriene-cyclohexadiene system also appears to involve a
tetraradicaloid structure, one component of which is an allylic system. These structures
can account for the major product types in both systems.
3
4
2 3 .
. . 2 . 5
1 . . 4 . .
1 6
1,2:3,4 pairing 1,3:2,4 pairing 1,5:4,6
1,4:2,3 pairing 1,2:5,6 pairing
pairing 1,6:4,5
pairing
These results, as well as those from cyclooctatetraene and 1,5-cyclooctadiene, indicate
that structures with isolated and allylic unpaired electrons play a key role in diene
186
T. D. R. Manning and P. J. Kropp, J. Am. Chem. Soc., 103, 889 (1981).