Page 1154 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1154
eV 1139
TOPIC 12.1
7 S 2
Computational
?
S 1 Interpretation of Diene
and Polyene
6 Photochemistry
5
4
T 1
3
?
2
1
0 S
0
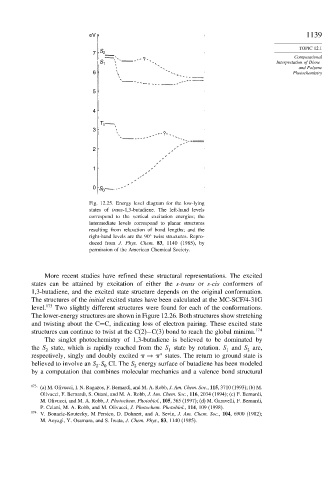
Fig. 12.25. Energy level diagram for the low-lying
states of trans-1,3-butadiene. The left-hand levels
correspond to the vertical excitation energies; the
intermediate levels correspond to planar structures
resulting from relaxation of bond lengths; and the
right-hand levels are the 90 twist structures. Repro-
duced from J. Phys. Chem. 83, 1140 (1985), by
permission of the American Chemical Society.
More recent studies have refined these structural representations. The excited
states can be attained by excitation of either the s-trans or s-cis conformers of
1,3-butadiene, and the excited state structure depends on the original conformation.
The structures of the initial excited states have been calculated at the MC-SCF/4-31G
level. 173 Two slightly different structures were found for each of the conformations.
The lower-energy structures are shown in Figure 12.26. Both structures show stretching
and twisting about the C=C, indicating loss of electron pairing. These excited state
structures can continue to twist at the C(2)−C(3) bond to reach the global minima. 174
The singlet photochemistry of 1,3-butadiene is believed to be dominated by
the S state, which is rapidly reached from the S state by rotation. S and S are,
1
2
2
1
∗
respectively, singly and doubly excited → states. The return to ground state is
believed to involve an S -S CI. The S energy surface of butadiene has been modeled
2 0 2
by a computation that combines molecular mechanics and a valence bond structural
173 (a) M. Olivucci, I. N. Ragazos, F. Bernardi, and M. A. Robb, J. Am. Chem. Soc., 115, 3710 (1993); (b) M.
Olivucci, F. Bernardi, S. Ottani, and M. A. Robb, J. Am. Chem. Soc., 116, 2034 (1994); (c) F. Bernardi,
M. Olivucci, and M. A. Robb, J. Photochem. Photobiol., 105, 365 (1997); (d) M. Garavelli, F. Bernardi,
P. Celani, M. A. Robb, and M. Olivucci, J. Photochem. Photobiol., 114, 109 (1998).
174
V. Bonacic-Koutecky, M Persico, D. Dohnert, and A. Sevin, J. Am. Chem. Soc., 104, 6900 (1982);
M. Aoyagi, Y. Osamura, and S. Iwata, J. Chem. Phys., 83, 1140 (1985).